Effect of Increasing Age on Brain Dysfunction in Cirrhosis
- PMID: 30619995
- PMCID: PMC6312655
- DOI: 10.1002/hep4.1286
Effect of Increasing Age on Brain Dysfunction in Cirrhosis
Abstract
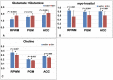
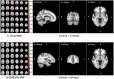
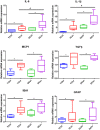
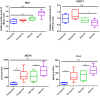
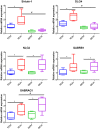
Patients with cirrhosis are growing older, which could have an impact on brain dysfunction beyond hepatic encephalopathy. Our aim was to study the effect of concomitant aging and cirrhosis on brain inflammation and degeneration using human and animal experiments. For the human study, age-matched patients with cirrhosis and controls between 65 and 85 years underwent cognitive testing, quality of life (QOL) assessment, and brain magnetic resonance (MR) spectroscopy and resting state functional MR imaging (rs-fMRI) analysis. Data were compared between groups. For the animal study, young (10-12 weeks) and old (1.5 years) C57BL/6 mice were given either CCl4 gavage to develop cirrhosis or a vehicle control and were followed for 12 weeks. Cortical messenger RNA (mRNA) expression of inflammatory mediators (interleukin [IL]-6, IL-1β, transforming growth factor β [TGF-β], and monocyte chemoattractant protein 1), sirtuin-1, and gamma-aminobutyric acid (GABA)-ergic synaptic plasticity (neuroligin-2 [NLG2], discs large homolog 4 [DLG4], GABA receptor, subunit gamma 1/subunit B1 [GABRG1/B1]) were analyzed and compared between younger/older control and cirrhotic mice. The human study included 46 subjects (23/group). Patients with cirrhosis had worse QOL and cognition. On MR spectroscopy, patients with cirrhosis had worse changes related to ammonia and lower N-acetyl aspartate, whereas rs-fMRI analysis revealed that these patients demonstrated functional connectivity changes in the frontoparietal cortical region compared to controls. Results of the animal study showed that older mice required lower CCl4 to reach cirrhosis. Older mice, especially with cirrhosis, demonstrated higher cortical inflammatory mRNA expression of IL-6, IL-1β, and TGF-β; higher glial and microglial activation; and lower sirtuin-1 expression compared to younger mice. Older mice also had lower expression of DLG4, an excitatory synaptic organizer, and higher NLG2 and GABRG1/B1 receptor expression, indicating a predominantly inhibitory synaptic organization. Conclusion: Aging modulates brain changes in cirrhosis; this can affect QOL, cognition, and brain connectivity. Cortical inflammation, microglial activation, and altered GABA-ergic synaptic plasticity could be contributory.
Figures
Similar articles
-
Evaluation of cognitivity, proinflammatory cytokines, and brain magnetic resonance imaging in minimal hepatic encephalopathy induced by cirrhosis and extrahepatic portal vein obstruction.J Gastroenterol Hepatol. 2016 Dec;31(12):1986-1994. doi: 10.1111/jgh.13427. J Gastroenterol Hepatol. 2016. PMID: 27119420
-
Hepatic Branch Vagotomy Modulates the Gut-Liver-Brain Axis in Murine Cirrhosis.Front Physiol. 2021 Jun 25;12:702646. doi: 10.3389/fphys.2021.702646. eCollection 2021. Front Physiol. 2021. PMID: 34248683 Free PMC article.
-
Brain inflammation induces post-synaptic changes during early synapse formation in adult-born hippocampal neurons.Exp Neurol. 2013 Dec;250:176-88. doi: 10.1016/j.expneurol.2013.09.005. Epub 2013 Sep 15. Exp Neurol. 2013. PMID: 24047952
-
Is there an association between peripheral immune markers and structural/functional neuroimaging findings?Prog Neuropsychopharmacol Biol Psychiatry. 2014 Jan 3;48:295-303. doi: 10.1016/j.pnpbp.2012.12.013. Epub 2013 Jan 11. Prog Neuropsychopharmacol Biol Psychiatry. 2014. PMID: 23313563 Review.
-
[Ammonia and GABA-ergic neurotransmission in pathogenesis of hepatic encephalopathy].Wiad Lek. 2003;56(11-12):560-3. Wiad Lek. 2003. PMID: 15058165 Review. Polish.
Cited by
-
The Association between Hepatic Encephalopathy and Diabetic Encephalopathy: The Brain-Liver Axis.Int J Mol Sci. 2021 Jan 5;22(1):463. doi: 10.3390/ijms22010463. Int J Mol Sci. 2021. PMID: 33466498 Free PMC article. Review.
-
A Possible Reversible Cause of Cognitive Impairment: Undiagnosed Cirrhosis and Potential Hepatic Encephalopathy in Patients with Dementia.Am J Med. 2024 Nov;137(11):1082-1087.e1. doi: 10.1016/j.amjmed.2024.06.014. Epub 2024 Jun 26. Am J Med. 2024. PMID: 38942345
-
2021 ISHEN guidelines on animal models of hepatic encephalopathy.Liver Int. 2021 Jul;41(7):1474-1488. doi: 10.1111/liv.14911. Epub 2021 May 11. Liver Int. 2021. PMID: 33900013 Free PMC article. Review.
-
Neuroinflammation in Murine Cirrhosis Is Dependent on the Gut Microbiome and Is Attenuated by Fecal Transplant.Hepatology. 2020 Feb;71(2):611-626. doi: 10.1002/hep.30827. Epub 2019 Aug 19. Hepatology. 2020. PMID: 31220352 Free PMC article.
-
Gut microbiome-brain-cirrhosis axis.Hepatology. 2024 Aug 1;80(2):465-485. doi: 10.1097/HEP.0000000000000344. Epub 2023 Mar 6. Hepatology. 2024. PMID: 36866864 Free PMC article. Review.
References
-
- Frith J, Jones D, Newton JL. Chronic liver disease in an ageing population. Age Ageing 2009;38:11‐18. - PubMed
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715‐735. - PubMed
-
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm‐aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244‐254. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

