Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study
- PMID: 30620669
- PMCID: PMC6528729
- DOI: 10.1200/JCO.18.00766
Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study
Abstract
Purpose: Treatment options are limited for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL). Tumor cells can exploit the programmed death-1 checkpoint pathway to evade immune surveillance. In the current study, we evaluated the efficacy and safety of programmed death-1 blockade by nivolumab in patients with relapsed/refractory DLBCL.
Methods: In this phase II, open-label study, patients with relapsed/refractory DLBCL who were ineligible for autologous hematopoietic cell transplantation (auto-HCT) or who had experienced failure with auto-HCT received nivolumab 3 mg/kg every 2 weeks. We assessed the efficacy and safety of nivolumab as well as genetic alterations of 9p24.1.
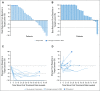
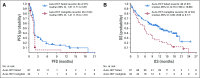
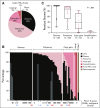
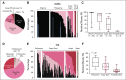
Results: Among 121 treated patients, patients in the auto-HCT-failed cohort (n = 87) received a median of four nivolumab doses and a median of three doses were administered to those in the auto-HCT-ineligible cohort (n = 34). At a median follow-up of 9 months in the auto-HCT-failed cohort and 6 months in the auto-HCT-ineligible cohort, independently assessed objective response rates were 10% and 3%, and median durations of response were 11 and 8 months, respectively. Median progression-free survival and overall survival were 1.9 and 12.2 months in the auto-HCT-failed cohort and 1.4 and 5.8 months in the auto-HCT-ineligible cohort respectively. All three patients with complete remission-3% of the auto-HCT-failed cohort-had durable response (11 or more, 14 or more, and 17 months). Treatment-related grade 3 and 4 adverse events were reported in 24% of patients. The most common were neutropenia (4%), thrombocytopenia (3%), and increased lipase (3%). Of all evaluable samples for 9p24.1 analysis, 16% exhibited low-level copy gain and 3% had amplification.
Conclusion: Nivolumab monotherapy is associated with a favorable safety profile but a low overall response rate among patients with DLBCL who are ineligible for auto-HCT or who experienced failure with auto-HCT. Genetic alterations of 9p24.1 are infrequent in DLBCL.
Trial registration: ClinicalTrials.gov NCT02038933.
Figures
References
-
- International Agency for Research on Cancer : World Cancer Report 2014: Diffuse Large B-cell Lymphoma. Geneva, Switzerland, WHO Press, 2014
-
- Friedberg JW: Relapsed/refractory diffuse large B-cell lymphoma. Hematology (Am Soc Hematol Educ Program) 2011:498-505, 2011 - PubMed
-
- National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin’s lymphomas, version 4.2014. https://www.nccn.org/about/nhl.pdf
-
- Nagle SJ, Woo K, Schuster SJ, et al. : Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol 88:890-894, 2013 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

