Augmentation of DMLS Biomimetic Dental Implants with Weight-Bearing Strut to Balance of Biologic and Mechanical Demands: From Bench to Animal
- PMID: 30621012
- PMCID: PMC6337105
- DOI: 10.3390/ma12010164
Augmentation of DMLS Biomimetic Dental Implants with Weight-Bearing Strut to Balance of Biologic and Mechanical Demands: From Bench to Animal
Abstract
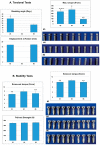
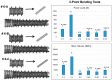
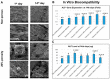
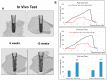
A mismatch of elastic modulus values could result in undesirable bone resorption around the dental implant. The objective of this study was to optimize direct metal laser sintering (DMLS)-manufactured Ti₆Al₄V dental implants' design, minimize elastic mismatch, allow for maximal bone ingrowth, and improve long-term fixation of the implant. In this study, DMLS dental implants with different morphological characteristics were fabricated. Three-point bending, torsional, and stability tests were performed to compare the mechanical properties of different designs. Improvement of the weaker design was attempted by augmentation with a longitudinal 3D-printed strut. The osseointegrative properties were evaluated. The results showed that the increase in porosity decreased the mechanical properties, while augmentation with a longitudinal weight-bearing strut can improve mechanical strength. Maximal alkaline phosphatase gene expression of MG63 cells attained on 60% porosity Ti₆Al₄V discs. In vivo experiments showed good incorporation of bone into the porous scaffolds of the DMLS dental implant, resulting in a higher pull-out strength. In summary, we introduced a new design concept by augmenting the implant with a longitudinal weight-bearing strut to achieve the ideal combination of high strength and low elastic modulus; our results showed that there is a chance to reach the balance of both biologic and mechanical demands.
Keywords: 3D-printing; Ti6Al4V; biomimetic; direct metal laser sintering; porous titanium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Yavari S.A., Wauthle R., van der Stok J., Riemslag A.C., Janssen M., Mulier M., Kruth J.P., Schrooten J., Weinans H., Zadpoor A.A. Fatigue behavior of porous biomaterials manufactured using selective laser melting. Mater. Sci. Eng. C Mater. Biol. Appl. 2013;33:4849–4858. doi: 10.1016/j.msec.2013.08.006. - DOI - PubMed
-
- Kirmanidou Y., Sidira M., Drosou M.E., Bennani V., Bakopoulou A., Tsouknidas A., Michailidis N., Michalakis K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. Biomed. Res. Int. 2016;2016:2908570. doi: 10.1155/2016/2908570. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

