Chimeric Antigen Receptor T-cell (CAR T) Therapy for Hematologic and Solid Malignancies: Efficacy and Safety-A Systematic Review with Meta-Analysis
- PMID: 30621018
- PMCID: PMC6356949
- DOI: 10.3390/cancers11010047
Chimeric Antigen Receptor T-cell (CAR T) Therapy for Hematologic and Solid Malignancies: Efficacy and Safety-A Systematic Review with Meta-Analysis
Abstract
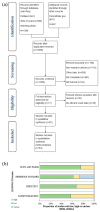
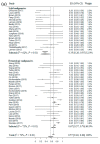
Chimeric antigen receptors T cells (CAR T) had been used for treating various tumor patients in clinic, and owned an incredible efficacy in part of malignancies. However, CAR T therapy remains controversial due to doubts about its efficacy and safety in the clinical treatment of various malignancies. A total of 997 tumor patients from 52 studies were included in this review. Eligible studies were searched and reviewed from the databases of PubMed, Web of Science, Wanfang and Clinicaltrials.gov. Then meta-analysis and subgroup analysis were used to investigate the overall response rate (ORR), complete response rate (CRR), common side effect rate (CSER) and relapse rate (RR) of CAR T therapy for patients in clinical researches, respectively. The results further confirmed that CAR T therapy had a higher response rate for hematologic malignancies. More importantly, CAR T therapy had a higher CSER in patients with hematologic malignancies, and it had a similar RR in patients with different malignancies. Cell cultured without the addition of IL-2 and total administration less than 10⁸ cells were recommended. This study offers a reference for future research regarding the application in solid and hematologic malignancies, side effects and relapse, and even the production processes of CAR T cells.
Keywords: chimeric antigen receptor T cell; meta-analysis; meta-regression analysis; relapse; response; side effect; subgroup analysis.
Conflict of interest statement
The authors declare no competing interests. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures








References
-
- Zhang T., Cao L., Xie J., Shi N., Zhang Z., Luo Z., Yue D., Zhang Z., Wang L., Han W., et al. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: A meta-analysis. Oncotarget. 2015;6:33961–33971. doi: 10.18632/oncotarget.5582. - DOI - PMC - PubMed
-
- Magee M.S., Snook A.E. Challenges to chimeric antigen receptor (CAR)-T cell therapy for cancer. Discov. Med. 2014;18:265–271. - PubMed
Publication types
Grants and funding
- 2017YFA0104301, 2016YFC0902700, 2017YFA0506002/the National Key R&D Research Program by Ministry of Science and Technology
- 81630092, 31801084, 81773099, 81570790, 81573338/the Chinese National Natural Sciences Foundation
- BZ2017018, BRA2017347, BK20171202/Jiangsu Province Science and Technology Innovation Committee
- JCYJ20160331152141936/Shenzhen Science and Technology Innovation Committee
- KQTD20140630165057031/Shenzhen Peacock Plan
LinkOut - more resources
Full Text Sources

