Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms
- PMID: 30621045
- PMCID: PMC6356449
- DOI: 10.3390/md17010032
Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms
Abstract
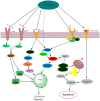
Fucoidan is a natural derived compound found in different species of brown algae and in some animals, that has gained attention for its anticancer properties. However, the exact mechanism of action is currently unknown. Therefore, this review will address fucoidans structure, the bioavailability, and all known different pathways affected by fucoidan, in order to formulate fucoidans structure and activity in relation to its anti-cancer mechanisms. The general bioactivity of fucoidan is difficult to establish due to factors like species-related structural diversity, growth conditions, and the extraction method. The main pathways influenced by fucoidan are the PI3K/AKT, the MAPK pathway, and the caspase pathway. PTEN seems to be important in the fucoidan-mediated effect on the AKT pathway. Furthermore, the interaction with VEGF, BMP, TGF-β, and estrogen receptors are discussed. Also, fucoidan as an adjunct seems to have beneficial effects, for both the enhanced effectiveness of chemotherapy and reduced toxicity in healthy cells. In conclusion, the multipotent character of fucoidan is promising in future anti-cancer treatment. However, there is a need for more specified studies of the structure⁻activity relationship of fucoidan from the most promising seaweed species.
Keywords: AKT; MAPK; brown algae; cancer; fucoidan; natural product; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Svejda B., Aguiriano-Moser V., Sturm S., Hoger H., Ingolic E., Siegl V., Stuppner H., Pfragner R. Anticancer Activity of Novel Plant Extracts from Trailliaedoxa gracilis (W. W. Smith & Forrest) in Human Carcinoid KRJ-I Cells. Anticancer Res. 2010;30:55–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

