Grape Seed Proanthocyanidin Extract Alleviates AflatoxinB₁-Induced Immunotoxicity and Oxidative Stress via Modulation of NF-κB and Nrf2 Signaling Pathways in Broilers
- PMID: 30621062
- PMCID: PMC6356337
- DOI: 10.3390/toxins11010023
Grape Seed Proanthocyanidin Extract Alleviates AflatoxinB₁-Induced Immunotoxicity and Oxidative Stress via Modulation of NF-κB and Nrf2 Signaling Pathways in Broilers
Abstract
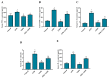
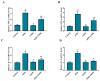
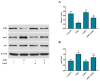
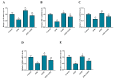
Aflatoxin B₁ (AFB₁) is a widely spread mycotoxin contaminates food and feed, causing severe oxidative stress damages and immunotoxicity. Grape seed proanthocyanidin (GSPE), a natural antioxidant with wide range of pharmacological and medicinal properties. The goal of the present study was to investigate the protective effects of GSPE against AFB₁-induced immunotoxicity and oxidative stress via NF-κB and Nrf2 signaling pathways in broiler chickens. For the experiment, 240 one-day old Cobb chicks were allocated into four dietary treatment groups of six replicates (10 birds per replicate): 1. Basal diet (control); 2. Basal diet + AFB₁ 1mg/kg contaminated corn (AFB₁); 3. Basal diet + GSPE 250 mg/kg (GSPE); 4. Basal diet + AFB₁ 1 mg/kg + GSPE 250 mg/kg (AFB₁ + GSPE). The results showed that GSPE significantly decreased serum inflammatory cytokines TNF-α, IFN-γ, IL-1β, IL-10, and IL-6 induced by AFB₁. Similarly, GSPE + AFB₁ treated group revealed a significant decrease in mRNA expressions of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β, and IL-6) in the splenic tissue compared to the AFB₁ treatment group. In addition, western blotting results manifested that GSPE treatment normalized the phosphorylation of nuclear factor kappa B (p65) and the degradation of IκBα protein induced by AFB₁. Furthermore, GSPE enhanced the antioxidant defense system through activating the nuclear factor-erythroid-2-related factor (Nrf2) signaling pathway. The mRNA and protein expression level of Nrf2 and its down streaming associated genes were noted up-regulated by the addition of GSPE, and down-regulated in the AFB₁ group. Taken together, GSPE alleviates AFB₁-induced immunotoxicity and oxidative damage by inhibiting the NF-κB and activating the Nrf2 signaling pathways in broiler chickens. Conclusively, our results suggest that GSPE could be considered as a potential natural agent for the prevention of AFB₁-induced immunotoxicity and oxidative damage.
Keywords: Aflatoxin B1; Broilers; Grape Seed Proanthocyanidin Extract; Immunotoxicity; NF-κB; Nrf2; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Méndez A. Decontamination of aflatoxin duckling feed with aqueous citric acid treatment. Anim. Feed Sci. Technol. 2007;135:249–262. doi: 10.1016/j.anifeedsci.2006.07.009. - DOI
-
- Herzallah S.M. Aflatoxin B1 residues in eggs and flesh of laying hens fed aflatoxin B1 contaminated diet. Am. J. Agric. Biol. Sci. 2013;8:156–161. doi: 10.3844/ajabssp.2013.156.161. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

