Small Multitarget Molecules Incorporating the Enone Moiety
- PMID: 30621100
- PMCID: PMC6337391
- DOI: 10.3390/molecules24010199
Small Multitarget Molecules Incorporating the Enone Moiety
Abstract
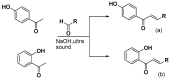
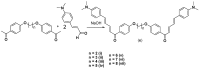
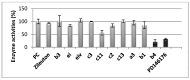
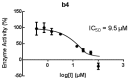
Chalcones represent a class of small drug/druglike molecules with different and multitarget biological activities. Small multi-target drugs have attracted considerable interest in the last decade due their advantages in the treatment of complex and multifactorial diseases, since "one drug-one target" therapies have failed in many cases to demonstrate clinical efficacy. In this context, we designed and synthesized potential new small multi-target agents with lipoxygenase (LOX), acetyl cholinesterase (AChE) and lipid peroxidation inhibitory activities, as well as antioxidant activity based on 2-/4- hydroxy-chalcones and the bis-etherified bis-chalcone skeleton. Furthermore, the synthesized molecules were evaluated for their cytotoxicity. Simple chalcone b4 presents significant inhibitory activity against the 15-human LOX with an IC50 value 9.5 µM, interesting anti-AChE activity, and anti-lipid peroxidation behavior. Bis-etherified chalcone c12 is the most potent inhibitor of AChE within the bis-etherified bis-chalcones followed by c11. Bis-chalcones c11 and c12 were found to combine anti-LOX, anti-AchE, and anti-lipid peroxidation activities. It seems that the anti-lipid peroxidation activity supports the anti-LOX activity for the significantly active bis-chalcones. Our circular dichroism (CD) study identified two structures capable of interfering with the aggregation process of Aβ. Compounds c2 and c4 display additional protective actions against Alzheimer's disease (AD) and add to the pleiotropic profile of the chalcone derivatives. Predicted results indicate that the majority of the compounds with the exception of c11 (144 Å) can cross the Blood Brain Barrier (BBB) and act in CNS. The results led us to propose new leads and to conclude that the presence of a double enone group supports better biological activities.
Keywords: Alzheimer; acetylcholinesterase inhibitors; bis-chalcones; bis-ethers; chalcones; lipoxygenase inhibitors; multitarget; β-amyloid peptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

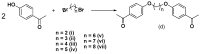
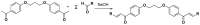

Similar articles
-
Simple chalcones and bis-chalcones ethers as possible pleiotropic agents.J Enzyme Inhib Med Chem. 2016;31(2):302-13. doi: 10.3109/14756366.2015.1021253. Epub 2015 Mar 23. J Enzyme Inhib Med Chem. 2016. PMID: 25798685
-
Ultrasound-assisted synthesis of novel chalcone, heterochalcone and bis-chalcone derivatives and the evaluation of their antioxidant properties and as acetylcholinesterase inhibitors.Bioorg Chem. 2019 Sep;90:103034. doi: 10.1016/j.bioorg.2019.103034. Epub 2019 Jun 8. Bioorg Chem. 2019. PMID: 31280015
-
Design, synthesis, in-silico and biological evaluation of novel chalcone-O-carbamate derivatives as multifunctional agents for the treatment of Alzheimer's disease.Eur J Med Chem. 2019 Sep 15;178:726-739. doi: 10.1016/j.ejmech.2019.06.026. Epub 2019 Jun 14. Eur J Med Chem. 2019. PMID: 31229875
-
Sulfonamide Derivatives: Recent Compounds with Potent Anti-alzheimer's Disease Activity.Cent Nerv Syst Agents Med Chem. 2024;24(1):82-104. doi: 10.2174/0118715249278489231128042135. Cent Nerv Syst Agents Med Chem. 2024. PMID: 38275073 Review.
-
Structural Modifications on Chalcone Framework for Developing New Class of Cholinesterase Inhibitors.Int J Mol Sci. 2022 Mar 14;23(6):3121. doi: 10.3390/ijms23063121. Int J Mol Sci. 2022. PMID: 35328542 Free PMC article. Review.
Cited by
-
2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities.Molecules. 2019 Dec 3;24(23):4411. doi: 10.3390/molecules24234411. Molecules. 2019. PMID: 31816866 Free PMC article.
-
High Impact: The Role of Promiscuous Binding Sites in Polypharmacology.Molecules. 2019 Jul 10;24(14):2529. doi: 10.3390/molecules24142529. Molecules. 2019. PMID: 31295958 Free PMC article.
-
Stereospecific Conversion of Boronic Esters into Enones using Methoxyallene: Application in the Total Synthesis of 10-Deoxymethynolide.Angew Chem Int Ed Engl. 2023 Dec 11;62(50):e202312054. doi: 10.1002/anie.202312054. Epub 2023 Nov 9. Angew Chem Int Ed Engl. 2023. PMID: 37877778 Free PMC article.
-
The Prophylactic and Multimodal Activity of Two Isatin Thiosemicarbazones against Alzheimer's Disease In Vitro.Brain Sci. 2022 Jun 19;12(6):806. doi: 10.3390/brainsci12060806. Brain Sci. 2022. PMID: 35741690 Free PMC article.
-
Novel Thiosemicarbazone Quantum Dots in the Treatment of Alzheimer's Disease Combining In Silico Models Using Fingerprints and Physicochemical Descriptors.ACS Omega. 2023 Mar 17;8(12):11076-11099. doi: 10.1021/acsomega.2c07934. eCollection 2023 Mar 28. ACS Omega. 2023. PMID: 37008140 Free PMC article.
References
-
- Gupta D., Jaina D.K., Trivedi P. Recent advances in chalcones as antiinfective agents. Int. J. Chem. Sci. 2010;8:649–654.
-
- Rahman M.A. Chalcone: A valuable insight into the recent advances and potential pharmacological activities. Chem. Sci. J. 2011;29:1–16. doi: 10.4172/2150-3494.1000021. - DOI
-
- Sinha S., Medhi B., Sehga R. Chalcones as an emerging lead molecule for antimalarial therapy: A review. J. Mod. Med. Chem. 2013;1:64–77.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

