Effect of Planarity of Aromatic Rings Appended to o-Carborane on Photophysical Properties: A Series of o-Carboranyl Compounds Based on 2-Phenylpyridine- and 2-(Benzo[b]thiophen-2-yl)pyridine
- PMID: 30621119
- PMCID: PMC6337515
- DOI: 10.3390/molecules24010201
Effect of Planarity of Aromatic Rings Appended to o-Carborane on Photophysical Properties: A Series of o-Carboranyl Compounds Based on 2-Phenylpyridine- and 2-(Benzo[b]thiophen-2-yl)pyridine
Abstract
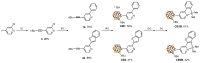
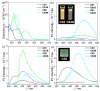
Herein, we investigated the effect of ring planarity by fully characterizing four pyridine-based o-carboranyl compounds. o-Carborane was introduced to the C4 position of the pyridine rings of 2-phenylpyridine and 2-(benzo[b]thiophen-2-yl)pyridine (CB1 and CB2, respectively), and the compounds were subsequently borylated to obtain the corresponding CN-chelated compounds CB1B and CB2B. Single-crystal X-ray diffraction analysis of the molecular structures of CB2 and CB2B confirmed that o-carborane is appended to the aryl moiety. In photoluminescence experiments, CB2, but not CB1, showed an intense emission, assignable to intramolecular charge transfer (ICT) transition between the aryl and o-carborane moieties, in both solution and film states. On the other hand, in both solution and film states, CB1B and CB2B demonstrated a strong emission, originating from π-π * transition in the aryl groups, that tailed off to 650 nm owing to the ICT transition. All intramolecular electronic transitions in these o-carboranyl compounds were verified by theoretical calculations. These results distinctly suggest that the planarity of the aryl groups have a decisive effect on the efficiency of the radiative decay due to the ICT transition.
Keywords: intramolecular charge transfer; o-carborane; radiative decay; structural fluctuation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
2-Phenylpyridine- and 2-(benzo[b]thiophen-2-yl)pyridine-based o-carboranyl compounds: impact of the structural formation of aromatic rings on photophysical properties.Dalton Trans. 2019 Jan 22;48(4):1467-1476. doi: 10.1039/c8dt04367a. Dalton Trans. 2019. PMID: 30631864
-
Impact of deboronation on the electronic characteristics of closo-o-carborane: intriguing photophysical changes in triazole-appended carboranyl luminophores.Dalton Trans. 2021 Mar 7;50(9):3207-3215. doi: 10.1039/d0dt04038j. Epub 2021 Feb 12. Dalton Trans. 2021. PMID: 33576753
-
Influence of Electronic Environment on the Radiative Efficiency of 9-Phenyl-9H-carbazole-Based ortho-Carboranyl Luminophores.Molecules. 2021 Mar 21;26(6):1763. doi: 10.3390/molecules26061763. Molecules. 2021. PMID: 33801078 Free PMC article.
-
Transition metal-carboryne complexes: synthesis, bonding, and reactivity.Acc Chem Res. 2011 Apr 19;44(4):299-309. doi: 10.1021/ar100156f. Epub 2011 Mar 11. Acc Chem Res. 2011. PMID: 21395260 Review.
-
Recent Progress in the Development of Solid-State Luminescent o-Carboranes with Stimuli Responsivity.Angew Chem Int Ed Engl. 2020 Jun 15;59(25):9841-9855. doi: 10.1002/anie.201916666. Epub 2020 Apr 1. Angew Chem Int Ed Engl. 2020. PMID: 32009291 Review.
Cited by
-
Deboronation-Induced Ratiometric Emission Variations of Terphenyl-Based Closo-o-Carboranyl Compounds: Applications to Fluoride-Sensing.Molecules. 2020 May 21;25(10):2413. doi: 10.3390/molecules25102413. Molecules. 2020. PMID: 32455846 Free PMC article.
-
Photophysical Properties of Spirobifluorene-Based o-Carboranyl Compounds Altered by Structurally Rotating the Carborane Cages.Molecules. 2019 Nov 15;24(22):4135. doi: 10.3390/molecules24224135. Molecules. 2019. PMID: 31731632 Free PMC article.
-
Alteration of intramolecular electronic transition via deboronation of carbazole-based o-carboranyl compound and intriguing 'turn-on' emissive variation.RSC Adv. 2021 Jul 7;11(39):24057-24064. doi: 10.1039/d1ra03716a. eCollection 2021 Jul 6. RSC Adv. 2021. PMID: 35479040 Free PMC article.
References
-
- Bregadze V.I. Dicarba-closo-dodecaboranes C2B10H12 and their derivatives. Chem. Rev. 1992;92:209–223. doi: 10.1021/cr00010a002. - DOI
-
- González-Campo A., Juárez-Pérez E.J., Viñas C., Boury B., Sillanpää R., Kivekäs R., Núñez R. Carboranyl Substituted Siloxanes and Octasilsesquioxanes: Synthesis, Characterization, and Reactivity. Macromolecules. 2008;41:8458–8466. doi: 10.1021/ma801483c. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

