Antiproliferative and Enzyme Docking Analysis of Engleromycin from Engleromyces goetzei
- PMID: 30621140
- PMCID: PMC6337443
- DOI: 10.3390/molecules24010166
Antiproliferative and Enzyme Docking Analysis of Engleromycin from Engleromyces goetzei
Abstract
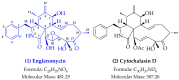
Engleromyces goetzei P. Henn. (E. goetzei) has been widely used as a traditional herb for many years in Kenya due to its diverse biological effects. Although engleromycin was first isolated from E. goetzei in 1980, its pharmacological activity is still unknown. In this study, engleromycin from E. goetzei was identified by spectroscopic analyses, and subsequently examined for its antiproliferative activity using human cancer cell lines of SGC-7901, HT-29, HeLa and A549. As a result, it was revealed that engleromycin strongly inhibited the growth of SGC-7901, HT-29, HeLa and A549 cells with IC50 values at 26.77 ± 1.69 µM, 7.73 ± 0.18 µM, 7.00 ± 0.12 µM and 3.14 ± 0.03 µM, respectively. The results of topoisomerase II (Top II) inhibition assay in vitro implied that engleromycin might be a Top II inhibitor. Further insights into the potential mechanism of antiproliferative activity displayed that engleromycin could dock into the binding pockets of Top II, like the clinical inhibitor doxorubicin, and then inhibit the biological activity of Top II. Taken together, our findings suggest that engleromycin has an anticancer potential, and may serve as a leading compound for the development of antitumor agents.
Keywords: Engleromyces goetzei; antiproliferative activities; engleromycin; molecular docking.
Conflict of interest statement
The authors declare no competing financial interests. These funders played no roles in the study design, data collection and analysis, and decision to publish.
Figures

Similar articles
-
Synthesis and biological evaluation of N-(carbobenzyloxy)-l-phenylalanine and N-(carbobenzyloxy)-l-aspartic acid-β-benzyl ester derivatives as potent topoisomerase IIα inhibitors.Bioorg Med Chem. 2017 Jun 15;25(12):3116-3126. doi: 10.1016/j.bmc.2017.03.065. Epub 2017 Apr 3. Bioorg Med Chem. 2017. PMID: 28462840
-
4β-amidotriazole linked podophyllotoxin congeners: DNA topoisomerase-IIα inhibition and potential anticancer agents for prostate cancer.Eur J Med Chem. 2018 Jan 20;144:595-611. doi: 10.1016/j.ejmech.2017.12.050. Epub 2017 Dec 14. Eur J Med Chem. 2018. PMID: 29289884
-
Synthesis of podophyllotoxin linked β-carboline congeners as potential anticancer agents and DNA topoisomerase II inhibitors.Eur J Med Chem. 2018 Jan 20;144:557-571. doi: 10.1016/j.ejmech.2017.12.055. Epub 2017 Dec 16. Eur J Med Chem. 2018. PMID: 29289881
-
Synthesis, crystal structure and antiproliferative activity of Cu(II) nalidixic acid-DACH conjugate: comparative in vitro DNA/RNA binding profile, cleavage activity and molecular docking studies.Eur J Med Chem. 2014 Jun 23;81:76-88. doi: 10.1016/j.ejmech.2014.04.080. Epub 2014 May 2. Eur J Med Chem. 2014. PMID: 24826817
-
Pharmacophore Hybridization To Discover Novel Topoisomerase II Poisons with Promising Antiproliferative Activity.J Med Chem. 2018 Feb 8;61(3):1375-1379. doi: 10.1021/acs.jmedchem.7b01388. Epub 2017 Nov 9. J Med Chem. 2018. PMID: 29077404
Cited by
-
Antioxidant, anti-inflammatory, and anticancer function of Engleromyces goetzei Henn aqueous extract on human intestinal Caco-2 cells treated with t-BHP.Food Sci Nutr. 2023 Apr 6;11(6):3450-3463. doi: 10.1002/fsn3.3335. eCollection 2023 Jun. Food Sci Nutr. 2023. PMID: 37324905 Free PMC article.
References
-
- Pedersen E.J., Larsen P., Boll P.M. Engleromycin, a new cytochalasan from Engleromyces goetzei hennings. Tetrahedron Lett. 1980;21:5079–5082. doi: 10.1016/S0040-4039(00)71139-7. - DOI
-
- Whalley M.A., Khalil A.M.A., Wei T.Z., Yao Y.J., Whalley A.J.S. A new species of Engleromyces from China, a second species in the genus. Mycotaxon. 2010;112:317–323. doi: 10.5248/112.317-. - DOI
-
- Kokwaro J.O. An African knowledge of ethnosystematics and its application to traditional medicine, with particular reference to the medicinal use of the fungus Engleromyces goetzei. Bothalia. 1983;14:237–243. doi: 10.4102/abc.v14i2.1168. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

