Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes
- PMID: 30621146
- PMCID: PMC6337705
- DOI: 10.3390/ijms20010160
Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes
Abstract
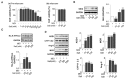
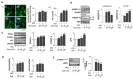
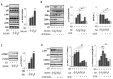
It is well known that high salt intake is associated with cardiovascular diseases including hypertension. However, the research on the mechanism of obesity due to high salt intake is rare. To evaluate the roles of salt on obesity prevalence, the gene expression of adipogenesis/lipogenesis and adipocytokines secretion according to adipocyte dysfunction were investigated in salt-loading adipocytes. High salt dose-dependently increased the expression of adipogenic/lipogenic genes, such as PPAR-γ, C/EBPα, SREBP1c, ACC, FAS, and aP2, but decreased the gene of lipolysis like AMPK, ultimately resulting in fat accumulation. With SIK-2 and Na⁺/K⁺-ATPase activation, salt increased the metabolites involved in the renin-angiotensin-aldosterone system (RAAS) such as ADD1, CYP11β2, and MCR. Increasing insulin dependent insulin receptor substrate (IRS)-signaling, resulting in the insulin resistance, mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and Akt-mTOR were activated but AMPK(Thr172) was depressed in salt-loading adipocytes. The expression of pro-inflammatory adipocytokines, TNFα, MCP-1, COX-2, IL-17A, IL-6, leptin, and leptin to adiponectin ratio (LAR) were dose-dependently increased by salt treatment. Using the inhibitors of MAPK/ERK, U0126, we found that the crosstalk among the signaling pathways of MAPK/ERK, Akt-mTOR, and the inflammatory adipogenesis can be the possible mechanism of salt-linked obesity. The possibilities of whether the defense mechanisms against high dose of intracellular salts provoke signaling for adipocytes differentiation or interact with surrounding tissues through other pathways will be explored in future research.
Keywords: Akt-mTOR; MAPK/ERK; adipogenesis; inflammatory cytokines; obesity; salt.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures


Similar articles
-
Inhibitory effect of sinigrin on adipocyte differentiation in 3T3-L1 cells: Involvement of AMPK and MAPK pathways.Biomed Pharmacother. 2018 Jun;102:670-680. doi: 10.1016/j.biopha.2018.03.124. Epub 2018 Apr 5. Biomed Pharmacother. 2018. PMID: 29604586
-
Heshouwu (Polygonum multiflorum Thunb.) ethanol extract suppresses pre-adipocytes differentiation in 3T3-L1 cells and adiposity in obese mice.Biomed Pharmacother. 2018 Oct;106:355-362. doi: 10.1016/j.biopha.2018.06.140. Epub 2018 Jul 11. Biomed Pharmacother. 2018. PMID: 29966981
-
Alpinia officinarum inhibits adipocyte differentiation and high-fat diet-induced obesity in mice through regulation of adipogenesis and lipogenesis.J Med Food. 2012 Nov;15(11):959-67. doi: 10.1089/jmf.2012.2286. J Med Food. 2012. PMID: 23126661
-
Human Protein Kinases and Obesity.Adv Exp Med Biol. 2017;960:111-134. doi: 10.1007/978-3-319-48382-5_5. Adv Exp Med Biol. 2017. PMID: 28585197 Review.
-
Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance.Inflammation. 2022 Feb;45(1):31-44. doi: 10.1007/s10753-021-01559-z. Epub 2021 Sep 18. Inflammation. 2022. PMID: 34536157 Free PMC article. Review.
Cited by
-
Single-Cell Landscape and a Macrophage Subset Enhancing Brown Adipocyte Function in Diabetes.Diabetes Metab J. 2024 Sep;48(5):885-900. doi: 10.4093/dmj.2023.0278. Epub 2024 May 29. Diabetes Metab J. 2024. PMID: 38853519 Free PMC article.
-
SPRY4 promotes adipogenic differentiation of human mesenchymal stem cells through the MEK-ERK1/2 signaling pathway.Adipocyte. 2022 Dec;11(1):588-600. doi: 10.1080/21623945.2022.2123097. Adipocyte. 2022. PMID: 36082406 Free PMC article.
-
Hypertonic saline mediates the NLRP3/IL-1β signaling axis in microglia to alleviate ischemic blood-brain barrier permeability by downregulating astrocyte-derived VEGF in rats.CNS Neurosci Ther. 2020 Oct;26(10):1045-1057. doi: 10.1111/cns.13427. Epub 2020 Jun 12. CNS Neurosci Ther. 2020. PMID: 32529750 Free PMC article.
-
The metabolic signature of salt intake: a cross-sectional analysis from the SCAPIS-study.Nutr Metab (Lond). 2025 Sep 2;22(1):104. doi: 10.1186/s12986-025-00997-y. Nutr Metab (Lond). 2025. PMID: 40898250 Free PMC article.
-
Hepatic Transcriptomics Reveals Reduced Lipogenesis in High-Salt Diet Mice.Genes (Basel). 2023 Apr 24;14(5):966. doi: 10.3390/genes14050966. Genes (Basel). 2023. PMID: 37239325 Free PMC article.
References
-
- Republic of Korea: Ministry of Health & Welfare 2010 Korean National Health and Nutrition Examination Survey (KNHANES) [(accessed on 3 December 2018)];2010 Available online: https://knhanes.cdc.go.kr.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

