Capillasterin A, a Novel Pyrano[2,3-f]chromene from the Australian Crinoid Capillaster multiradiatus
- PMID: 30621172
- PMCID: PMC6356231
- DOI: 10.3390/md17010026
Capillasterin A, a Novel Pyrano[2,3-f]chromene from the Australian Crinoid Capillaster multiradiatus
Abstract
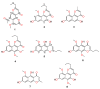
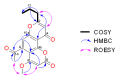
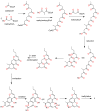
Capillasterin A (1), a novel pyrano[2,3-f]chromene, together with seven known naphthopyrones including comaparvin (2), TMC-256C1 (3), 6-methoxycomaparvin-5- methyl ether (4), 5,8-dihydroxy-6-methoxy-2-propyl-4H-naphtho[2,3-b]pyran-4-one (5), 5,8-dihydroxy-6,10-dimethoxy-2-propyl-4H-naphtho[2,3-b]pyran-4-one (6), TMC-256A1 (7) and 6-methoxycomaparvin (8) were isolated from an EtOH/H₂O extract from the Australian crinoid Capillaster multiradiatus. The structures of all the compounds were determined by detailed spectroscopic (1D/2D NMR and MS) data analysis. This is the first report of a natural product that contains the pyrano[2,3-f]chromene skeleton. Compounds 2⁻6 were observed to display moderate inhibition of in vitro HIV-1 replication in a T cell line with EC50 values ranging from 7.5 to 25.5 µM without concomitant cytotoxicity.
Keywords: Capillaster multiradiatus; Crinoid; HIV-1; capillasterin A; feather star; naphthopyrones; pyrano[2,3-f]chromene.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Inhibition of ABCG2-mediated drug efflux by naphthopyrones from marine crinoids.Bioorg Med Chem Lett. 2010 Jul 1;20(13):3848-50. doi: 10.1016/j.bmcl.2010.05.057. Epub 2010 May 21. Bioorg Med Chem Lett. 2010. PMID: 20627559 Free PMC article.
-
Anthraquinone and Butenolide Constituents from the Crinoid Capillaster multiradiatus.Chem Pharm Bull (Tokyo). 2018 Nov 1;66(11):1023-1026. doi: 10.1248/cpb.c18-00472. Epub 2018 Aug 21. Chem Pharm Bull (Tokyo). 2018. PMID: 30135325
-
Sulfated Naphthopyrones and Anthraquinones from the Vietnamese Crinoid Comanthus delicata.Chem Pharm Bull (Tokyo). 2022;70(5):408-412. doi: 10.1248/cpb.c21-00904. Chem Pharm Bull (Tokyo). 2022. PMID: 35491198
-
NF-kappaB-inhibiting naphthopyrones from the Fijian echinoderm Comanthus parvicirrus.J Nat Prod. 2008 Jan;71(1):106-11. doi: 10.1021/np070290y. Epub 2007 Dec 19. J Nat Prod. 2008. PMID: 18088098
-
Synthesis and pharmacological properties of polysubstituted 2-amino-4H-pyran-3-carbonitrile derivatives.Mol Divers. 2020 Nov;24(4):1385-1431. doi: 10.1007/s11030-019-09994-9. Epub 2019 Sep 25. Mol Divers. 2020. PMID: 31555954 Review.
Cited by
-
Ten-Year Research Update Review: Antiviral Activities from Marine Organisms.Biomolecules. 2020 Jul 7;10(7):1007. doi: 10.3390/biom10071007. Biomolecules. 2020. PMID: 32645994 Free PMC article. Review.
-
Fatty Acids of Echinoderms: Diversity, Current Applications and Future Opportunities.Mar Drugs. 2022 Dec 27;21(1):21. doi: 10.3390/md21010021. Mar Drugs. 2022. PMID: 36662194 Free PMC article. Review.
-
Marine-Derived Bioactive Metabolites as a Potential Therapeutic Intervention in Managing Viral Diseases: Insights from the SARS-CoV-2 In Silico and Pre-Clinical Studies.Pharmaceuticals (Basel). 2024 Mar 1;17(3):328. doi: 10.3390/ph17030328. Pharmaceuticals (Basel). 2024. PMID: 38543114 Free PMC article. Review.
-
Phanogracilins A-C, New Bibenzochromenones of Crinoid Phanogenia gracilis (Hartlaub, 1890).Biomolecules. 2024 Jan 26;14(2):151. doi: 10.3390/biom14020151. Biomolecules. 2024. PMID: 38397388 Free PMC article.
References
-
- Wätjen W., Ebada S.S., Bergermann A., Chovolou Y., Totzke F., Kubbutat M.H.G., Lin W., Proksch P. Cytotoxic effects of the anthraquinone derivatives 1′-deoxyrhodoptilometrin and (S)-(−)-rhodoptilometrin isolated from the marine echinoderm Comanthus sp. Arch. Toxicol. 2017;91:1485–1495. doi: 10.1007/s00204-016-1787-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous