Targeting DNA Double-Strand Break Repair Pathways to Improve Radiotherapy Response
- PMID: 30621219
- PMCID: PMC6356315
- DOI: 10.3390/genes10010025
Targeting DNA Double-Strand Break Repair Pathways to Improve Radiotherapy Response
Abstract
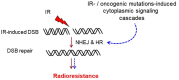
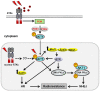
More than half of cancer patients receive radiotherapy as a part of their cancer treatment. DNA double-strand breaks (DSBs) are considered as the most lethal form of DNA damage and a primary cause of cell death and are induced by ionizing radiation (IR) during radiotherapy. Many malignant cells carry multiple genetic and epigenetic aberrations that may interfere with essential DSB repair pathways. Additionally, exposure to IR induces the activation of a multicomponent signal transduction network known as DNA damage response (DDR). DDR initiates cell cycle checkpoints and induces DSB repair in the nucleus by non-homologous end joining (NHEJ) or homologous recombination (HR). The canonical DSB repair pathways function in both normal and tumor cells. Thus, normal-tissue toxicity may limit the targeting of the components of these two pathways as a therapeutic approach in combination with radiotherapy. The DSB repair pathways are also stimulated through cytoplasmic signaling pathways. These signaling cascades are often upregulated in tumor cells harboring mutations or the overexpression of certain cellular oncogenes, e.g., receptor tyrosine kinases, PIK3CA and RAS. Targeting such cytoplasmic signaling pathways seems to be a more specific approach to blocking DSB repair in tumor cells. In this review, a brief overview of cytoplasmic signaling pathways that have been reported to stimulate DSB repair is provided. The state of the art of targeting these pathways will be discussed. A greater understanding of the underlying signaling pathways involved in DSB repair may provide valuable insights that will help to design new strategies to improve treatment outcomes in combination with radiotherapy.
Keywords: double-strand break repair; ionizing radiation; molecular targeting; radiosensitization; signal transduction.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

