Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques
- PMID: 30621309
- PMCID: PMC6359585
- DOI: 10.3390/pharmaceutics11010021
Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques
Abstract
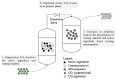
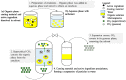
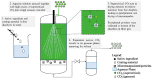
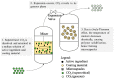
The unique properties of supercritical fluids, in particular supercritical carbon dioxide (CO₂), provide numerous opportunities for the development of processes for pharmaceutical applications. One of the potential applications for pharmaceuticals includes microencapsulation and nanoencapsulation for drug delivery purposes. Supercritical CO₂ processes allow the design and control of particle size, as well as drug loading by utilizing the tunable properties of supercritical CO2 at different operating conditions (flow ratio, temperature, pressures, etc.). This review aims to provide a comprehensive overview of the processes and techniques using supercritical fluid processing based on the supercritical properties, the role of supercritical carbon dioxide during the process, and the mechanism of formulation production for each process discussed. The considerations for equipment configurations to achieve the various processes described and the mechanisms behind the representative processes such as RESS (rapid expansion of supercritical solutions), SAS (supercritical antisolvent), SFEE (supercritical fluid extraction of emulsions), PGSS (particles from gas-saturated solutions), drying, and polymer foaming will be explained via schematic representation. More recent developments such as fluidized bed coating using supercritical CO₂ as the fluidizing and drying medium, the supercritical CO₂ spray drying of aqueous solutions, as well as the production of microporous drug releasing devices via foaming, will be highlighted in this review. Development and strategies to control and optimize the particle morphology, drug loading, and yield from the major processes will also be discussed.
Keywords: microencapsulation; microporous foam; supercritical anti-solvent; supercritical carbon dioxide; supercritical drying.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ginty P.J., Whitaker M.J., Shakesheff K.M., Howdle S.M. Drug delivery goes supercritical. Mater. Today. 2005;8:42–48. doi: 10.1016/S1369-7021(05)71036-1. - DOI
-
- Nuchuchua O., Nejadnik M.R., Goulooze S.C., Lješković N.J., Every H.A., Jiskoot W. Characterization of drug delivery particles produced by supercritical carbon dioxide technologies. J. Supercrit. Fluids. 2017;128:244–262. doi: 10.1016/j.supflu.2017.06.002. - DOI
-
- Fahim T.K., Zaidul I.S.M., Abu Bakar M.R., Salim U.M., Awang M.B., Sahena F., Jalal K.C.A., Sharif K.M., Sohrab M.H. Particle formation and micronization using non-conventional techniques—Review. Chem. Eng. Process. Process Intensif. 2014;86:47–52. doi: 10.1016/j.cep.2014.10.009. - DOI
Publication types
LinkOut - more resources
Full Text Sources

