Cardiomyocyte Progenitor Cells as a Functional Gene Delivery Vehicle for Long-Term Biological Pacing
- PMID: 30621310
- PMCID: PMC6337610
- DOI: 10.3390/molecules24010181
Cardiomyocyte Progenitor Cells as a Functional Gene Delivery Vehicle for Long-Term Biological Pacing
Abstract
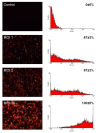
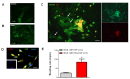
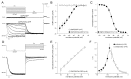
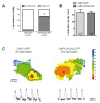
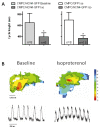
Sustained pacemaker function is a challenge in biological pacemaker engineering. Human cardiomyocyte progenitor cells (CMPCs) have exhibited extended survival in the heart after transplantation. We studied whether lentivirally transduced CMPCs that express the pacemaker current If (encoded by HCN4) can be used as functional gene delivery vehicle in biological pacing. Human CMPCs were isolated from fetal hearts using magnetic beads coated with Sca-1 antibody, cultured in nondifferentiating conditions, and transduced with a green fluorescent protein (GFP)- or HCN4-GFP-expressing lentivirus. A patch-clamp analysis showed a large hyperpolarization-activated, time-dependent inward current (-20 pA/pF at -140 mV, n = 14) with properties typical of If in HCN4-GFP-expressing CMPCs. Gap-junctional coupling between CMPCs and neonatal rat ventricular myocytes (NRVMs) was demonstrated by efficient dye transfer and changes in spontaneous beating activity. In organ explant cultures, the number of preparations showing spontaneous beating activity increased from 6.3% in CMPC/GFP-injected preparations to 68.2% in CMPC/HCN4-GFP-injected preparations (P < 0.05). Furthermore, in CMPC/HCN4-GFP-injected preparations, isoproterenol induced a significant reduction in cycle lengths from 648 ± 169 to 392 ± 71 ms (P < 0.05). In sum, CMPCs expressing HCN4-GFP functionally couple to NRVMs and induce physiologically controlled pacemaker activity and may therefore provide an attractive delivery platform for sustained pacemaker function.
Keywords: HCN channels; cell therapy; gene therapy; pacemakers; progenitor cells.
Conflict of interest statement
H.L.T. and G.J.J.B. report ownership interest in PacingCure B. V. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Engineering physiologically controlled pacemaker cells with lentiviral HCN4 gene transfer.J Gene Med. 2008 May;10(5):487-97. doi: 10.1002/jgm.1172. J Gene Med. 2008. PMID: 18383475
-
Pacemaker cell characteristics of differentiated and HCN4-transduced human mesenchymal stem cells.Life Sci. 2019 Sep 1;232:116620. doi: 10.1016/j.lfs.2019.116620. Epub 2019 Jul 7. Life Sci. 2019. PMID: 31291594
-
Dominant-negative suppression of HCN channels markedly reduces the native pacemaker current I(f) and undermines spontaneous beating of neonatal cardiomyocytes.Circulation. 2003 Jan 28;107(3):485-9. doi: 10.1161/01.cir.0000045672.32920.cb. Circulation. 2003. PMID: 12551875
-
Pacemaker activity of the human sinoatrial node: effects of HCN4 mutations on the hyperpolarization-activated current.Europace. 2014 Mar;16(3):384-95. doi: 10.1093/europace/eut348. Europace. 2014. PMID: 24569893 Review.
-
Human cardiomyocyte progenitor cells: a short history of nearly everything.J Cell Mol Med. 2012 Aug;16(8):1669-73. doi: 10.1111/j.1582-4934.2012.01535.x. J Cell Mol Med. 2012. PMID: 22260290 Free PMC article. Review.
Cited by
-
Conversion of Unmodified Stem Cells to Pacemaker Cells by Overexpression of Key Developmental Genes.Cells. 2023 May 13;12(10):1381. doi: 10.3390/cells12101381. Cells. 2023. PMID: 37408215 Free PMC article.
-
Toward Biological Pacing by Cellular Delivery of Hcn2/SkM1.Front Physiol. 2021 Jan 6;11:588679. doi: 10.3389/fphys.2020.588679. eCollection 2020. Front Physiol. 2021. PMID: 33488393 Free PMC article.
-
Biological pacemaker: from biological experiments to computational simulation.J Zhejiang Univ Sci B. 2020 Jul;21(7):524-536. doi: 10.1631/jzus.B1900632. J Zhejiang Univ Sci B. 2020. PMID: 32633107 Free PMC article. Review.
-
Isoproterenol pre-treatment improve the therapeutic efficacy of cardiosphere-derived cells transplantation for myocardial infarction.J Thorac Dis. 2023 Jun 30;15(6):3089-3105. doi: 10.21037/jtd-22-1593. Epub 2023 May 10. J Thorac Dis. 2023. PMID: 37426146 Free PMC article.
-
Editorial: Cardiac Pacemaking in Health and Disease: From Genes to Function.Front Physiol. 2022 May 30;13:913506. doi: 10.3389/fphys.2022.913506. eCollection 2022. Front Physiol. 2022. PMID: 35711314 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

