Catalytic Conversion of Carbon Dioxide through C-N Bond Formation
- PMID: 30621311
- PMCID: PMC6337678
- DOI: 10.3390/molecules24010182
Catalytic Conversion of Carbon Dioxide through C-N Bond Formation
Abstract
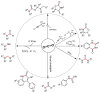
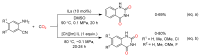
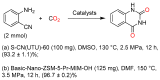
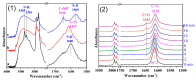
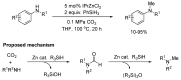
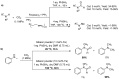
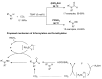
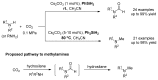
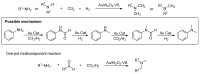
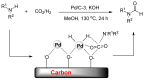
From the viewpoint of green chemistry and sustainable development, it is of great significance to synthesize chemicals from CO₂ as C₁ source through C-N bond formation. During the past several decade years, many studies on C-N bond formation reaction were involved, and many efforts have been made on the theory. Nevertheless, several great challenges such as thermodynamic limitation, low catalytic efficiency and selectivity, and high pressure etc. are still suffered. Herein, recent advances are highlighted on the development of catalytic methods for chemical fixation of CO₂ to various chemicals through C-N bond formation. Meanwhile, the catalytic systems (metal and metal-free catalysis), strategies and catalytic mechanism are summarized and discussed in detail. Besides, this review also covers some novel synthetic strategies to urethanes based on amines and CO₂. Finally, the regulatory strategies on functionalization of CO₂ for N-methylation/N-formylation of amines with phenylsilane and heterogeneous catalysis N-methylation of amines with CO₂ and H₂ are emphasized.
Keywords: C-N bond formation; carbon dioxide utilization; chemicals; hydrogenation; reaction mechanism; synthetic methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


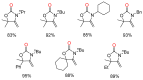













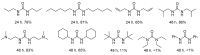





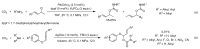



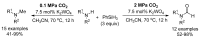







Similar articles
-
N-formylation and N-methylation of amines using metal-free N-heterocyclic carbene catalysts and CO2 as carbon source.Nat Protoc. 2017 Feb;12(2):417-428. doi: 10.1038/nprot.2016.175. Epub 2017 Jan 26. Nat Protoc. 2017. PMID: 28125103
-
Metal-free heterogeneous catalysis for sustainable chemistry.ChemSusChem. 2010 Feb 22;3(2):169-80. doi: 10.1002/cssc.200900180. ChemSusChem. 2010. PMID: 20127789 Review.
-
A New Energy-Saving Catalytic System: Carbon Dioxide Activation by a Metal/Carbon Catalyst.ChemSusChem. 2017 Sep 22;10(18):3671-3678. doi: 10.1002/cssc.201701283. Epub 2017 Sep 12. ChemSusChem. 2017. PMID: 28834353
-
Cu(I)/Ionic Liquids Promote the Conversion of Carbon Dioxide into Oxazolidinones at Room Temperature.Molecules. 2019 Mar 29;24(7):1241. doi: 10.3390/molecules24071241. Molecules. 2019. PMID: 30934963 Free PMC article.
-
Sustainable Catalyst-free N-formylation using CO2 as a Carbon Source.Curr Org Synth. 2022 Mar 3;19(2):187-196. doi: 10.2174/1570179418666211022160149. Curr Org Synth. 2022. PMID: 34719366 Review.
Cited by
-
Catalytic CO2 Reduction with Boron- and Aluminum Hydrides.ChemCatChem. 2019 Nov 7;11(21):5275-5281. doi: 10.1002/cctc.201901255. Epub 2019 Sep 30. ChemCatChem. 2019. PMID: 31894189 Free PMC article.
-
Advances in Palladium-Catalyzed Carboxylation Reactions.Molecules. 2022 Jan 1;27(1):262. doi: 10.3390/molecules27010262. Molecules. 2022. PMID: 35011494 Free PMC article. Review.
-
Endogenous melatonin involved in plant salt response by impacting auxin signaling.Plant Cell Rep. 2024 Jan 11;43(2):33. doi: 10.1007/s00299-023-03097-4. Plant Cell Rep. 2024. PMID: 38200226
-
Continuous Formation of Limonene Carbonates in Supercritical Carbon Dioxide.Org Process Res Dev. 2022 Oct 21;26(10):2799-2810. doi: 10.1021/acs.oprd.2c00143. Epub 2022 Sep 16. Org Process Res Dev. 2022. PMID: 36311380 Free PMC article. Review.
-
Thermochemical Properties of Synthesized Urea from Recovered Ammonia and Carbon Dioxide in Well-Ordered Nanospaces of Hollow Silica Spheres.ACS Omega. 2023 Dec 20;9(1):714-718. doi: 10.1021/acsomega.3c06534. eCollection 2024 Jan 9. ACS Omega. 2023. PMID: 38222630 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

