Overview of HCV Life Cycle with a Special Focus on Current and Possible Future Antiviral Targets
- PMID: 30621318
- PMCID: PMC6356578
- DOI: 10.3390/v11010030
Overview of HCV Life Cycle with a Special Focus on Current and Possible Future Antiviral Targets
Abstract
Hepatitis C infection is the leading cause of liver diseases worldwide and a major health concern that affects an estimated 3% of the global population. Novel therapies available since 2014 and 2017 are very efficient and the WHO considers HCV eradication possible by the year 2030. These treatments are based on the so-called direct acting antivirals (DAAs) that have been developed through research efforts by academia and industry since the 1990s. After a brief overview of the HCV life cycle, we describe here the functions of the different targets of current DAAs, the mode of action of these DAAs and potential future inhibitors.
Keywords: DAA; HCV; antiviral targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
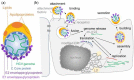
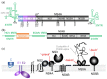
References
-
- International Committee on Taxonomy of Viruses (ICTV) [(accessed on 22 July 2018)]; Available online: https://talk.ictvonline.org/ictv_wikis/flaviviridae/w/sg_flavi/56/hcv-cl....
-
- Hoofnagle J.H., Mullen K.D., Jones D.B., Rustgi V., Di Bisceglie A., Peters M., Waggoner J.G., Park Y., Jones E.A. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N. Engl. J. Med. 1986;315:1575–1578. doi: 10.1056/NEJM198612183152503. - DOI - PubMed
-
- Poynard T., Marcellin P., Lee S.S., Niederau C., Minuk G.S., Ideo G., Bain V., Heathcote J., Zeuzem S., Trepo C., et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–1432. doi: 10.1016/S0140-6736(98)07124-4. - DOI - PubMed
-
- Manns M.P., McHutchison J.G., Gordon S.C., Rustgi V.K., Shiffman M., Reindollar R., Goodman Z.D., Koury K., Ling M., Albrecht J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

