Ontogenetic Changes in Auxin Biosynthesis and Distribution Determine the Organogenic Activity of the Shoot Apical Meristem in pin1 Mutants
- PMID: 30621327
- PMCID: PMC6337202
- DOI: 10.3390/ijms20010180
Ontogenetic Changes in Auxin Biosynthesis and Distribution Determine the Organogenic Activity of the Shoot Apical Meristem in pin1 Mutants
Abstract
In the shoot apical meristem (SAM) of Arabidopsis, PIN1-dependent polar auxin transport (PAT) regulates two crucial developmental processes: organogenesis and vascular system formation. However, the knockout mutation in the PIN1 gene does not fully inhibit these two processes. Therefore, we investigated a potential source of auxin for organogenesis and vascularization during inflorescence stem development. We analyzed auxin distribution in wild-type (WT) and pin1 mutant plants using a refined protocol of auxin immunolocalization; auxin activity, with the response reporter pDR5:GFP; and expression of auxin biosynthesis genes YUC1 and YUC4. Our results revealed that regardless of the functionality of PIN1-mediated PAT, auxin is present in the SAM and vascular strands. In WT plants, auxin always accumulates in all cells of the SAM, whereas in pin1 mutants, its localization within the SAM changes ontogenetically and is related to changes in the structure of the vascular system, organogenic activity of SAM, and expression levels of YUC1 and YUC4 genes. Our findings indicate that the presence of auxin in the meristem of pin1 mutants is an outcome of at least two PIN1-independent mechanisms: acropetal auxin transport from differentiated tissues with the use of vascular strands and auxin biosynthesis within the SAM.
Keywords: Arabidopsis; PAT; SAM; YUC genes; auxin immunolocalization; organogenesis; pin1 mutant; vascular system; xylem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
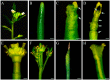

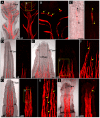
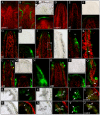
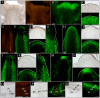

References
-
- Dengler N.G. The shoot apical meristem and development of vascular architecture. Can. J. Bot. 2006;84:1660–1671. doi: 10.1139/b06-126. - DOI
-
- Steeves T.A., Sussex I.A. Patterns in Plant Development. Cambridge University Press; Cambridge, UK: 1989.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

