Synthesis of diN-Substituted Glycyl-Phenylalanine Derivatives by Using Ugi Four Component Reaction and Their Potential as Acetylcholinesterase Inhibitors
- PMID: 30621344
- PMCID: PMC6337627
- DOI: 10.3390/molecules24010189
Synthesis of diN-Substituted Glycyl-Phenylalanine Derivatives by Using Ugi Four Component Reaction and Their Potential as Acetylcholinesterase Inhibitors
Abstract
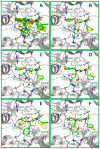
Ugi four component reaction (Ugi-4CR) isocyanide-based multicomponent reactions were used to synthesize diN-substituted glycyl-phenylalanine (diNsGF) derivatives. All of the synthesized compounds were characterized by spectroscopic and spectrometric techniques. In order to evaluate potential biological applications, the synthesized compounds were tested in computational models that predict the bioactivity of organic molecules by using only bi-dimensional molecular information. The diNsGF derivatives were predicted as cholinesterase inhibitors. Experimentally, all of the synthesized diNsGF derivatives showed moderate inhibitory activities against acetylcholinesterase (AChE) and poor activities against butyrylcholinesterase (BuChE). Compound 7a has significant activity and selectivity against AChE, which reveals that the diNsGF scaffold could be improved to reach novel candidates by combining other chemical components of the Ugi-4CR in a high-throughput combinatorial screening experiment. Molecular docking experiments of diNsGF derivatives inside AChE suggest that these compounds placed the phenylalanine group at the peripheral site of AChE. The orientations and chemical interactions of diNsGF derivatives were analyzed, and the changeable groups were identified for future exploration of novel candidates that could lead to the improvement of diNsGF derivative inhibitory activities.
Keywords: N-substituted phenylalanine derivatives; Ugi-4CR; acetylcholinesterase inhibitors; molecular docking; multicomponent reactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis of some new 3-coumaranone and coumarin derivatives as dual inhibitors of acetyl- and butyrylcholinesterase.Arch Pharm (Weinheim). 2013 Aug;346(8):577-87. doi: 10.1002/ardp.201300080. Epub 2013 Jul 15. Arch Pharm (Weinheim). 2013. PMID: 23852709
-
Design, synthesis, biological evaluation and docking study of 5-oxo-4,5-dihydropyrano[3,2-c]chromene derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors.Eur J Med Chem. 2013 Oct;68:260-9. doi: 10.1016/j.ejmech.2013.07.038. Epub 2013 Aug 11. Eur J Med Chem. 2013. PMID: 23988409
-
Evaluation of Novel Dual Acetyl- and Butyrylcholinesterase Inhibitors as Potential Anti-Alzheimer's Disease Agents Using Pharmacophore, 3D-QSAR, and Molecular Docking Approaches.Molecules. 2017 Jul 26;22(8):1254. doi: 10.3390/molecules22081254. Molecules. 2017. PMID: 28933746 Free PMC article.
-
Library-to-Library Synthesis of Highly Substituted α-Aminomethyl Tetrazoles via Ugi Reaction.ACS Comb Sci. 2018 Feb 12;20(2):70-74. doi: 10.1021/acscombsci.7b00137. Epub 2018 Jan 4. ACS Comb Sci. 2018. PMID: 29215263 Free PMC article. Review.
-
Ugi Four-Component Reactions Using Alternative Reactants.Molecules. 2023 Feb 8;28(4):1642. doi: 10.3390/molecules28041642. Molecules. 2023. PMID: 36838630 Free PMC article. Review.
References
-
- Slobbe P., Ruijter E., Orru R.V.A. Recent applications of multicomponent reactions in medicinal chemistry. Med. Chem. Commun. 2012;3:1189–1218. doi: 10.1039/c2md20089a. - DOI
-
- Ahmadi T., Mohammadi Ziarani G., Gholamzadeh P., Mollabagher H. Recent advances in asymmetric multicomponent reactions (AMCRs) Tetrahedron Asymmetry. 2017;28:708–724. doi: 10.1016/j.tetasy.2017.04.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

