Crocin ameliorates hepatic steatosis through activation of AMPK signaling in db/db mice
- PMID: 30621686
- PMCID: PMC6325828
- DOI: 10.1186/s12944-018-0955-6
Crocin ameliorates hepatic steatosis through activation of AMPK signaling in db/db mice
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) is closely linked to obesity, type 2 diabetes and other metabolic disorders worldwide. Crocin is a carotenoid compound possessing various pharmacological activities. In the present study, we aimed to investigate the effect on fatty liver under diabetic and obese condition and to examine the possible role of AMP-activated protein kinase (AMPK) signaling.
Methods: db/db mice were administrated with crocin and injected with LV-shAMPK or its negative control lentivirus. Metabolic dysfunction, lipogenesis and fatty acid-oxidation in liver were evaluated.
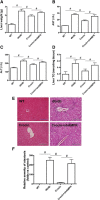
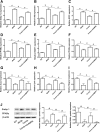
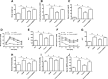
Results: In db/db mice, we found that oral administration of crocin significantly upregulated the phosphorylation of AMPK and downregulated the phosphorylation of mTOR in liver. Crocin reduced liver weight, serum levels of alanine aminotransferase, alanine aminotransferase, and liver triglyceride content, and attenuated morphological injury of liver in db/db mice. Crocin inhibited the mRNA expression of lipogenesis-associated genes, including sterol regulatory element binding protein-1c, peroxisome proliferator-activated receptor γ, fatty acid synthase, stearoyl-CoA desaturase 1, and diacylglycerol acyltransferase 1, and increased the mRNA expression of genes involved in the regulation of β-oxidation of fatty acids, including PPARα, acyl-CoA oxidase 1, carnitine palmitoyltransferase 1, and 3-hydroxy-3-methylglutaryl-CoA synthase 2. Moreover, treatment of crocin resulted in a amelioration of general metabolic disorder, as evidenced by decreased fasting blood glucose, reduced serum levels of insulin, triglyceride, total cholesterol, and non-esterified fatty acid, and improved glucose intolerance. Crocin-induced protective effects against fatty liver and metabolic disorder were significantly blocked by lentivirus-mediated downregulation of AMPK.
Conclusions: The results suggest that crocin can inhibit lipogenesis and promote β-oxidation of fatty acids through activation of AMPK, leading to improvement of fatty liver and metabolic dysfunction. Therefore, crocin may be a potential promising option for the clinical treatment for NAFLD and associated metabolic diseases.
Keywords: AMPK; NAFLD; antioxidant; crocin; type 2 diabetes.
Conflict of interest statement
Ethics approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology and in accordance with ARRIVE and NIH guidelines for animal welfare.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
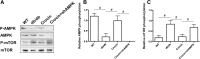
Similar articles
-
LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway.World J Gastroenterol. 2019 Dec 7;25(45):6607-6618. doi: 10.3748/wjg.v25.i45.6607. World J Gastroenterol. 2019. PMID: 31832001 Free PMC article.
-
P2Y2R Deficiency Ameliorates Hepatic Steatosis by Reducing Lipogenesis and Enhancing Fatty Acid β-Oxidation through AMPK and PGC-1α Induction in High-Fat Diet-Fed Mice.Int J Mol Sci. 2021 May 24;22(11):5528. doi: 10.3390/ijms22115528. Int J Mol Sci. 2021. PMID: 34073834 Free PMC article.
-
Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease.J Nutr Biochem. 2015 Apr;26(4):337-44. doi: 10.1016/j.jnutbio.2014.10.016. Epub 2014 Dec 5. J Nutr Biochem. 2015. PMID: 25622859 Free PMC article.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
Cited by
-
Crocin Improves Insulin Sensitivity and Ameliorates Adiposity by Regulating AMPK-CDK5-PPARγ Signaling.Biomed Res Int. 2020 Jun 6;2020:9136282. doi: 10.1155/2020/9136282. eCollection 2020. Biomed Res Int. 2020. PMID: 32596392 Free PMC article.
-
A Review on Drug Induced Obesity and Rodent Experimental Models of Obesity in Animals.Maedica (Bucur). 2022 Sep;17(3):706-713. doi: 10.26574/maedica.2022.17.3.706. Maedica (Bucur). 2022. PMID: 36540593 Free PMC article.
-
Modulating effects of crocin on lipids and lipoproteins: Mechanisms and potential benefits.Heliyon. 2024 Apr 3;10(7):e28837. doi: 10.1016/j.heliyon.2024.e28837. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38617922 Free PMC article. Review.
-
The effect of Crocin on sperm parameters disorders induced by peripheral administration of nitrosamines in male rats.J Med Life. 2022 Mar;15(3):362-367. doi: 10.25122/jml-2019-0144. J Med Life. 2022. PMID: 35450005 Free PMC article.
-
Crocin prevents metabolic syndrome in rats via enhancing PPAR-gamma and AMPK.Saudi J Biol Sci. 2020 May;27(5):1310-1316. doi: 10.1016/j.sjbs.2020.01.004. Epub 2020 Jan 27. Saudi J Biol Sci. 2020. PMID: 32346340 Free PMC article.
References
-
- Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. - DOI - PMC - PubMed
-
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

