Treatment response to indacaterol/glycopyrronium versus salmeterol/fluticasone in exacerbating COPD patients by gender: a post-hoc analysis in the FLAME study
- PMID: 30621717
- PMCID: PMC6325763
- DOI: 10.1186/s12931-019-0972-7
Treatment response to indacaterol/glycopyrronium versus salmeterol/fluticasone in exacerbating COPD patients by gender: a post-hoc analysis in the FLAME study
Abstract
Background: The burden of chronic obstructive lung disease (COPD) is increasing in women, with recent evidence suggesting gender differences in disease characteristics and potentially in treatment outcomes.
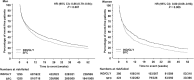
Methods: FLAME was a 52-week randomized controlled trial in patients with severe-to-very-severe COPD and a history of exacerbations. In this post-hoc analysis, gender-based baseline differences and treatment outcomes between indacaterol/glycopyrronium 110/50 μg once daily (IND/GLY) and salmeterol/fluticasone 50/500 twice daily (SFC) were assessed in terms of rate of exacerbations, time-to-first exacerbation, lung function, health status, and rescue medication use.
Results: This post-hoc analysis included 2557 men and 805 women. Baseline characteristics differed between genders, with women being younger, having better lung function and more often experiencing ≥2 exacerbations in the previous year. Compared with SFC, IND/GLY treatment was associated with reductions in the annualized rates of moderate/severe exacerbations (rate ratio [95% CI]: 0.81 [0.73-0.91], 0.89 [0.74-1.07] in men and women, respectively). Similarly, time-to-first moderate/severe exacerbation was also delayed (hazard ratio [95% CI]: 0.79 [0.70-0.89] and 0.76 [0.63-0.91] in men and women, respectively). Results were similar for all (mild/moderate/severe) exacerbations. Improvements in lung function, health status and rescue medication use with IND/GLY vs SFC were comparable between men and women. The smaller sample size for women may account for some observed discrepancies in treatment responses.
Conclusions: Although there were gender differences in baseline characteristics, IND/GLY demonstrated similar trends for exacerbation prevention and lung function improvement in men and women with moderate-to-very-severe COPD and a history of exacerbations compared with SFC. Small differences in the effects seen between genders may be attributed to the different sizes of the two groups and need to be further evaluated in randomized trials that are appropriately powered for gender analysis.
Trial registration: Post hoc analysis of the FLAME study. ClinicalTrials.gov number: NCT01782326 . Registered 1 February 2013.
Keywords: Chronic obstructive pulmonary disease; Exacerbation reduction; Gender; Indacaterol/glycopyrronium; Lung function.
Conflict of interest statement
Ethics approval and consent to participate
The FLAME study protocol and all amendments were reviewed by an Independent Ethics Committee or Institutional Review Board for each center in each country (Japan, Korea, Latvia, Lithuania, Mexico, Netherlands, Norway, Philippines, Poland, Portugal, Romania, Russia, Slovakia, South Africa, Spain, Sweden, Taiwan, Turkey, United Kingdom, Thailand, Croatia, Serbia, Argentina, Austria, Belgium, Bulgaria, Canada, Chile, China, Colombia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Guatemala, Hong Kong, Hungary, Iceland, India, Italy). The study was conducted as per the guidelines in the Declaration of Helsinki and Good Clinical Practice guidelines.
Consent for publication
Not applicable.
Competing interests
Jadwiga A. Wedzicha (JAW) has not received any speaker or consulting fees since January 2015. She has received research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Johnson and Johnson. Dave Singh (DS) reports grants and personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Glenmark, Johnson and Johnson, Merck, NAPP, Novartis, Pfizer, Takeda, Teva, Theravance, and Verona, and personal fees from Genentech and Skyepharma. Ioanna Tsiligianni (IT) has received honoraria for educational activities, speaking engagements and advisory boards from Boehringer Ingelheim, Novartis, AstraZeneca and GlaxoSmithKline. Christine Jenkins (CJ) reports personal fees from Novartis Pharma AG, during the conduct of the study; grants and personal fees from GlaxoSmithKline, personal fees from AstraZeneca, Boehringer Ingelheim, Menarini, outside the submitted work. Robert Fogel (RF), Sebastian Fucile (SF), Steven Shen (SS), Pankaj Goyal (PG), Karen Mezzi (KM) are employees and shareholders of the analysis sponsor, Novartis. Konstantinos Kostikas (KK) was an employee of Novartis Pharma AG at the time of the conduct of this study. None of the authors received any compensation for the development of the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures