Comparison of dexmedetomidine vs. remifentanil combined with sevoflurane during radiofrequency ablation of hepatocellular carcinoma: a randomized controlled trial
- PMID: 30621749
- PMCID: PMC6326039
- DOI: 10.1186/s13063-018-3010-z
Comparison of dexmedetomidine vs. remifentanil combined with sevoflurane during radiofrequency ablation of hepatocellular carcinoma: a randomized controlled trial
Abstract
Background: Remifentanil is widely used for ultrasound-guided percutaneous radiofrequency ablation (RFA) of small hepatocellular carcinoma (HCC). We determined whether dexmedetomidine could be an alternative to remifentanil for RFA of HCC under general anesthesia with sevoflurane.
Methods: We prospectively randomized patients scheduled to undergo RFA for HCC to a dexmedetomidine (DEX) group or remifentanil (REMI) group (47 patients each). In the DEX group, a bolus infusion (0.4 μg kg- 1) was started 15 min before anesthesia induction and continued at 0.2 μg kg- 1 h- 1 until 10 min before the end of surgery. In the REMI group, 3 μg kg- 1 h- 1 of remifentanil was administered from 15 min before anesthesia induction to the end of the surgery. The primary endpoint was postoperative pain intensity. Secondary endpoints included analgesic requirement, postoperative liver function, patient comfort, and hemodynamic changes. Group allocation was concealed from patients and data analysts but not from anesthesiologists.
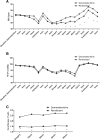
Results: Postoperative pain intensity, analgesic consumption, comfort, liver function, and time to emergence and extubation did not differ between the two groups. Heart rate, but not mean arterial pressure, was significantly lower in the DEX group than in the REMI group, at 1 min after intubation and from 30 min after the start of the surgery until anesthesia recovery. Sevoflurane concentration and dosage were significantly lower in the DEX group than in the REMI group.
Conclusion: During RFA for HCC, low-dose dexmedetomidine reduced the heart rate and need for inhalational anesthetics, without exacerbating postoperative discomfort or liver dysfunction. Although it did not exhibit outstanding advantages over remifentanil in terms of pain management, dexmedetomidine could be a safe alternative adjuvant for RFA under sevoflurane anesthesia.
Trial registration: Chinese Clinical Trial Registry, ChiCTR-OPC-15006613 . Registered on 16 June 2015.
Keywords: Catheter ablation; Dexmedetomidine; Pain measurement.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was provided by the ethics committee of the Third Affiliated Hospital of Sun Yat Sen University, Guangzhou, China on 5 May 2015.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
[Study of comparing dexmedetomidine and remifentanil for conscious sedation during radiofrequency ablation of hepatocellular carcinoma].Zhonghua Yi Xue Za Zhi. 2018 Feb 27;98(8):576-580. doi: 10.3760/cma.j.issn.0376-2491.2018.08.004. Zhonghua Yi Xue Za Zhi. 2018. PMID: 29534384 Clinical Trial. Chinese.
-
Efficacy of Single-Dose Dexmedetomidine Combined with Low-Dose Remifentanil Infusion for Cough Suppression Compared to High-Dose Remifentanil Infusion: A Randomized, Controlled, Non-Inferiority Trial.Int J Med Sci. 2019 Jan 24;16(3):376-383. doi: 10.7150/ijms.30227. eCollection 2019. Int J Med Sci. 2019. PMID: 30911271 Free PMC article. Clinical Trial.
-
A Randomized Comparison of Remifentanil Target-Controlled Infusion Versus Dexmedetomidine Single-Dose Administration: A Better Method for Smooth Recovery From General Sevoflurane Anesthesia.Am J Ther. 2016 May-Jun;23(3):e690-6. doi: 10.1097/01.mjt.0000433939.84373.2d. Am J Ther. 2016. PMID: 24100256 Clinical Trial.
-
Effects of dexmedetomidine on sevoflurane requirement for 50% excellent tracheal intubation in children: a randomized, double-blind comparison.Paediatr Anaesth. 2014 Sep;24(9):987-93. doi: 10.1111/pan.12430. Epub 2014 May 14. Paediatr Anaesth. 2014. PMID: 24823715 Review.
-
Effect of desflurane-remifentanil or sevoflurane-remifentanil on early recovery in elderly patients: a meta-analysis of randomized controlled trials.Pharmazie. 2019 Apr 1;74(4):201-205. doi: 10.1691/ph.2019.8935. Pharmazie. 2019. PMID: 30940302 Review.
Cited by
-
Exploring the impact of circRNAs on cancer glycolysis: Insights into tumor progression and therapeutic strategies.Noncoding RNA Res. 2024 May 5;9(3):970-994. doi: 10.1016/j.ncrna.2024.05.001. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38770106 Free PMC article. Review.
-
Combined sevoflurane-dexmedetomidine and nerve blockade on post-surgical serum oxidative stress biomarker levels in thyroid cancer patients.World J Clin Cases. 2022 Apr 6;10(10):3027-3034. doi: 10.12998/wjcc.v10.i10.3027. World J Clin Cases. 2022. PMID: 35647114 Free PMC article.
-
Comparative Efficacy of Dexmedetomidine and Remifentanil in Reducing Postoperative Pain and Opioid Use: A Systematic Review.Cureus. 2025 Feb 27;17(2):e79759. doi: 10.7759/cureus.79759. eCollection 2025 Feb. Cureus. 2025. PMID: 40028430 Free PMC article. Review.
-
Remifentanil reduces the proliferation, migration and invasion of HCC cells via lncRNA NBR2/miR-650/TIMP3 axis.Int J Exp Pathol. 2022 Apr;103(2):44-53. doi: 10.1111/iep.12429. Epub 2022 Feb 13. Int J Exp Pathol. 2022. PMID: 35156240 Free PMC article.
-
Safety and efficacy of pentazocine-midazolam combination for pain and anxiety relief in radiofrequency ablation therapy for hepatocellular carcinoma.JGH Open. 2022 Jul 17;6(8):569-576. doi: 10.1002/jgh3.12788. eCollection 2022 Aug. JGH Open. 2022. PMID: 35928702 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

