Population genetics of Anopheles funestus, the African malaria vector, Kenya
- PMID: 30621756
- PMCID: PMC6323828
- DOI: 10.1186/s13071-018-3252-3
Population genetics of Anopheles funestus, the African malaria vector, Kenya
Abstract
Background: Anopheles funestus is among the major malaria vectors in Kenya and sub-Saharan Africa and has been recently implicated in persistent malaria transmission. However, its ecology and genetic diversity remain poorly understood in Kenya.
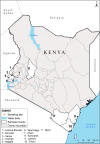
Methods: Using 16 microsatellite loci, we examined the genetic structure of An. funestus sampled from 11 locations (n = 426 individuals) across a wide geographical range in Kenya spanning coastal, western and Rift Valley areas.
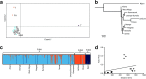
Results: Kenyan An. funestus resolved as three genetically distinct clusters. The largest cluster (FUN1) broadly included samples from western and Rift Valley areas of Kenya with two clusters identified from coastal Kenya (FUN2 and FUN3), not previously reported. Geographical distance had no effect on population differentiation of An. funestus. We found a significant variation in the mean Plasmodium infectivity between the clusters (χ2 = 12.1, df = 2, P = 0.002) and proportional to the malaria prevalence in the different risk zones of Kenya. Notably, there was variation in estimated effective population sizes between the clusters, suggesting possible differential impact of anti-vector interventions in represented areas.
Conclusions: Heterogeneity among Kenyan populations of An. funestus will impact malaria vector control with practical implications for the development of gene-drive technologies. The difference in Plasmodium infectivity and effective population size between the clusters could suggest potential variation in phenotypic characteristics relating to competence or insecticide resistance. This is worth examining in future studies.
Keywords: Anopheles funestus; Kenya; Malaria risk zones; Malaria vector; Microsatellites; Population genetics.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was acquired from the Kenya Medical Research Institute (KEMRI) Ethics Review Committee (Non-SSC Protocol #388 and Non-SSC Protocol #310). Sampling was done after receiving informed consent from village elders and household heads.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- WHO . World malaria report 2017. Geneva: World Health Organization; 2017.
-
- National Malaria Control Programme (NMCP) Kenya National Bureau of Statistics (KNBS), and ICF International . Kenya Malaria Indicator Survey 2015. Nairobi, Kenya, and Rockville, MD, USA: NMCP, KNBS, and ICF International; 2016. p. 165.
-
- President’s Malaria Initiative Kenya . Malaria Operational Plan Fy 2017. Washington: US Department of State, US Agency for International Development, Centers for Disease Control and Prevention; 2017.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

