Evaluation of an intensive education program on the treatment of tobacco-use disorder for pharmacists: a study protocol for a randomized controlled trial
- PMID: 30621772
- PMCID: PMC6324165
- DOI: 10.1186/s13063-018-3068-7
Evaluation of an intensive education program on the treatment of tobacco-use disorder for pharmacists: a study protocol for a randomized controlled trial
Abstract
Background: Tobacco use is presently responsible for the death of over seven million people across the world. In Qatar, it is one of the main causes of premature deaths and preventable diseases. To reduce tobacco use, Qatar has ratified the World Health Organization (WHO)'s Framework Convention on Tobacco Control (FCTC) and has implemented many tobacco-control initiatives. In spite of these measures, tobacco use is still considered a public health threat in Qatar. Pharmacists practicing in retail/community pharmacy settings are often the first port of call for individuals requiring general health advice. Evidence has proven that they have a pivotal role in health promotion and disease prevention including tobacco cessation. However, pharmacists in Qatar are not actively involved in tobacco control and many have not received any education or training about smoking cessation counseling in the past. In an effort to build the capacity of pharmacists towards tobacco control in Qatar, the aim of the proposed study is to design, implement, and evaluate an intensive education program on tobacco dependence treatment for pharmacists in Qatar.
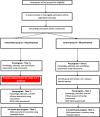
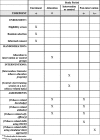
Methods/design: The study will be a prospective randomized controlled trial comparing an intensive tobacco-related education program versus non-tobacco-related training on pharmacists' tobacco-use-related knowledge, attitudes, self-efficacy, and skills. Community pharmacists practicing in Qatar will be eligible for participation in the study. A random sample of pharmacists will be selected for participation. Consenting participants will be randomly allocated to intervention or control groups. Participants in the intervention group will receive an intensive education program delivered by a multi-disciplinary group of educators, researchers, and clinicians with expertise in tobacco cessation. A short didactic session on a non-tobacco-related topic will be delivered to pharmacists in the control group. The study has two primary outcomes: post-intervention tobacco-related knowledge and post-intervention skills for tobacco cessation assessed using a multiple-choice-based evaluation instrument and an Objective Structured Clinical Examination (OSCE), respectively. The secondary study outcomes are post-intervention attitudes towards tobacco cessation and self-efficacy in tobacco-cessation interventions assessed using a survey instrument. An additional secondary study outcome is the post-intervention performance difference in relation to tobacco-cessation skills in the practice setting assessed using the simulated client approach.
Discussion: If demonstrated to be effective, this education program will be considered as a model that Qatar and the Middle East region can apply to overcome the burden of tobacco-use disorder.
Trial registration: ClinicalTrials.gov, ID: NCT03518476 . Registered on 8 May 2018. Version 1/22 June 2018.
Keywords: Education program; Pharmacist; Qatar; Smoking cessation; Tobacco control.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol and all associated measurement tools, consent forms, and recruitment procedures were reviewed and approved by Qatar University (QU) Institutional Review Board (QU-IRB 906-E/18). The participants will sign written informed consents to participate in this study.
Consent for publication
Not applicable. The manuscript does not report individual data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- The World Health Organization: Tobacco. 2018. http://www.who.int/news-room/fact-sheets/detail/tobacco. Accessed 12 May 2018.
-
- Centers for Disease Control and Prevention: Smoking and Tobacco Use. 2018. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.... Accessed 12 May 2018.
-
- The World Health Organization: Tobacco Free Initiative fact sheet about health benefits of smoking cessation. 2018. http://www.who.int/tobacco/quitting/benefits/en/. Accessed 12 May 2018.
-
- Centers for Disease Control and Prevention: Quitting Smoking. 2017. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/quitti.... Accessed 12 May 2018.
-
- U.S. Department of Health and Human Services: Treating tobacco use and dependence: 2008 update. 2008. https://www.ahrq.gov/professionals/clinicians-providers/guidelines-recom.... Accessed 12 May 2018.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous