Wellbeing intervention for chronic kidney disease (WICKD): a randomised controlled trial study protocol
- PMID: 30621791
- PMCID: PMC6325814
- DOI: 10.1186/s40359-018-0264-x
Wellbeing intervention for chronic kidney disease (WICKD): a randomised controlled trial study protocol
Abstract
Background: Incidence of end stage kidney disease (ESKD) for Indigenous Australians is especially high in remote and very remote areas of Australia (18 and 20 times the rate of comparable non-Indigenous people). Relocating away from family and country for treatment, adjusting to life with a chronic condition and time lost to dialysis cause grief and sadness which have immense impact on quality of life and challenges treatment adherence. We describe the first randomised controlled trial to address both chronic disease and mental health in Indigenous people with ESKD, which is the first to test the effectiveness of a culturally adapted e-mental health intervention in this population. It builds on an existing program of mental health research with demonstrated efficacy - the Aboriginal and Islander Mental Health Initiative (AIMhi) - to test the newly developed electronic motivational care planning (MCP) therapy - the AIMhi Stay Strong App.
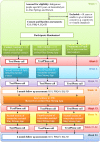
Methods: This is a 3-arm, waitlist, single-blind randomised controlled trial testing the efficacy of the Stay Strong App in improving psychological distress, depressive symptoms, quality of life and treatment adherence among Indigenous clients undergoing haemodialysis for ESKD in Alice Springs and Darwin with follow up over two periods of 3 months (total of 6 months observation). The study compares the efficacy of MCP using the AIMhi Stay Strong App with two control groups (control app intervention and treatment as usual) on participant-reported psychological distress (the primary outcome) using the Kessler Distress Scale (K10); depressive symptoms using the adapted Patient Health Questionnaire (PHQ-9); quality of life using the EuroQoL instrument (EQ5D) and adherence to dialysis treatment planning through file audit. Participants are randomised to receive MCP either at baseline (early treatment) or after 3 months (delayed treatment). The study also examines the cost effectiveness of this therapy in this setting through examination of health care service utilisation across groups during the first 3 months.
Discussion: This project will contribute much needed evidence on the efficacy of an electronic wellbeing intervention for Indigenous people with ESKD - a group in which distress is likely to be unacceptably high, yet relatively untreated.
Trial registration: Australian New Zealand Clinical Trial Registry; ACTRN12617000249358 ; Date registered: 17/02/2017.
Keywords: E-mental health; Indigenous; Kidney disease; Renal; Wellbeing.
Conflict of interest statement
Ethics approval and consent to participate
This study has been approved by the Central Australian Human Research Ethics Committee (CAHREC No: HREC-16-406) and the Human Research Ethics Committee (HREC) for the NT Department of Health and Menzies School of Health Research (HREC-16-2599), including an Aboriginal subcommittee. Fully informed oral consent is obtained from all participants prior to participation and in line with HREC approval as the study includes participants from diverse languages and cultures. Adverse events and Severe Adverse events are monitored by an Independent Safety Monitor and reported to the above two ethics committees within 72 h of becoming aware of them. Protocol amendments are submitted and approved by the above ethics committees prior to implementing changes.
Consent for publication
Not applicable.
Competing interests
KD, TN and DK developed the Stay Strong App which is a paid App. Menzies receives the limited revenue from App sales which is used for maintenance of the App. JH, AC, MS, KH, SB, CS, and SM have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Australian Institute of Health and Welfare . Aboriginal and Torres Strait islander health performance framework 2012: detailed analyses. Canberra: Australian Institute of Health and Welfare; 2013.
-
- Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pelllegrini F, Saglimbene V, Logroscino G, Fishbane S, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84:179–191. doi: 10.1038/ki.2013.77. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous