Clonal-level lineage commitment pathways of hematopoietic stem cells in vivo
- PMID: 30622181
- PMCID: PMC6347684
- DOI: 10.1073/pnas.1801480116
Clonal-level lineage commitment pathways of hematopoietic stem cells in vivo
Abstract
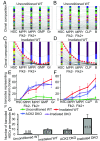
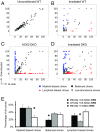
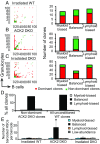
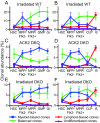
While the aggregate differentiation of the hematopoietic stem cell (HSC) population has been extensively studied, little is known about the lineage commitment process of individual HSC clones. Here, we provide lineage commitment maps of HSC clones under homeostasis and after perturbations of the endogenous hematopoietic system. Under homeostasis, all donor-derived HSC clones regenerate blood homogeneously throughout all measured stages and lineages of hematopoiesis. In contrast, after the hematopoietic system has been perturbed by irradiation or by an antagonistic anti-ckit antibody, only a small fraction of donor-derived HSC clones differentiate. Some of these clones dominantly expand and exhibit lineage bias. We identified the cellular origins of clonal dominance and lineage bias and uncovered the lineage commitment pathways that lead HSC clones to different levels of self-renewal and blood production under various transplantation conditions. This study reveals surprising alterations in HSC fate decisions directed by conditioning and identifies the key hematopoiesis stages that may be manipulated to control blood production and balance.
Keywords: clonal tracking; hematopoietic stem cell; lineage commitment; radiation; transplantation preconditioning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

