RNA Structure Duplication in the Dengue Virus 3' UTR: Redundancy or Host Specificity?
- PMID: 30622191
- PMCID: PMC6325252
- DOI: 10.1128/mBio.02506-18
RNA Structure Duplication in the Dengue Virus 3' UTR: Redundancy or Host Specificity?
Abstract
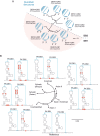
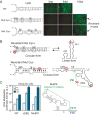
Flaviviruses include a diverse group of medically important viruses that cycle between mosquitoes and humans. During this natural process of switching hosts, each species imposes different selective forces on the viral population. Using dengue virus (DENV) as model, we found that paralogous RNA structures originating from duplications in the viral 3' untranslated region (UTR) are under different selective pressures in the two hosts. These RNA structures, known as dumbbells (DB1 and DB2), were originally proposed to be enhancers of viral replication. Analysis of viruses obtained from infected mosquitoes showed selection of mutations that mapped in DB2. Recombinant viruses carrying the identified variations confirmed that these mutations greatly increase viral replication in mosquito cells, with low or no impact in human cells. Use of viruses lacking each of the DB structures revealed opposite viral phenotypes. While deletion of DB1 reduced viral replication about 10-fold, viruses lacking DB2 displayed a great increase of fitness in mosquitoes, confirming a functional diversification of these similar RNA elements. Mechanistic analysis indicated that DB1 and DB2 differentially modulate viral genome cyclization and RNA replication. We found that a pseudoknot formed within DB2 competes with long-range RNA-RNA interactions that are necessary for minus-strand RNA synthesis. Our results support a model in which a functional diversification of duplicated RNA elements in the viral 3' UTR is driven by host-specific requirements. This study provides new ideas for understanding molecular aspects of the evolution of RNA viruses that naturally jump between different species.IMPORTANCE Flaviviruses constitute the most relevant group of arthropod-transmitted viruses, including important human pathogens such as the dengue, Zika, yellow fever, and West Nile viruses. The natural alternation of these viruses between vertebrate and invertebrate hosts shapes the viral genome population, which leads to selection of different viral variants with potential implications for epidemiological fitness and pathogenesis. However, the selective forces and mechanisms acting on the viral RNA during host adaptation are still largely unknown. Here, we found that two almost identical tandem RNA structures present at the viral 3' untranslated region are under different selective pressures in the two hosts. Mechanistic studies indicated that the two RNA elements, known as dumbbells, contain sequences that overlap essential RNA cyclization elements involved in viral RNA synthesis. The data support a model in which the duplicated RNA structures differentially evolved to accommodate distinct functions for viral replication in the two hosts.
Keywords: RNA virus; RNA virus evolution; RNA-RNA interactions; dengue virus; flavivirus; host adaptation; viral RNA structures.
Copyright © 2019 de Borba et al.
Figures





References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi:10.1038/nature12060. - DOI - PMC - PubMed
-
- Iglesias N, Byk L, Gamarnik A. 2014. Molecular biology of dengue virus, p 334–364. In Gubler DJ, Ooi EE, Vasudevan S, Farrar J (ed), Dengue and dengue hemorrhagic fever, 2nd ed CABI, Wallingford, United Kingdom.
-
- Manokaran G, Finol E, Wang C, Gunaratne J, Bahl J, Ong EZ, Tan HC, Sessions OM, Ward AM, Gubler DJ, Harris E, Garcia-Blanco MA, Ooi EE. 2015. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 350:217–221. doi:10.1126/science.aab3369. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
