N-degron and C-degron pathways of protein degradation
- PMID: 30622213
- PMCID: PMC6329975
- DOI: 10.1073/pnas.1816596116
N-degron and C-degron pathways of protein degradation
Abstract
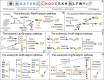
This perspective is partly review and partly proposal. N-degrons and C-degrons are degradation signals whose main determinants are, respectively, the N-terminal and C-terminal residues of cellular proteins. N-degrons and C-degrons include, to varying extents, adjoining sequence motifs, and also internal lysine residues that function as polyubiquitylation sites. Discovered in 1986, N-degrons were the first degradation signals in short-lived proteins. A particularly large set of C-degrons was discovered in 2018. We describe multifunctional proteolytic systems that target N-degrons and C-degrons. We also propose to denote these systems as "N-degron pathways" and "C-degron pathways." The former notation replaces the earlier name "N-end rule pathways." The term "N-end rule" was introduced 33 years ago, when only some N-terminal residues were thought to be destabilizing. However, studies over the last three decades have shown that all 20 amino acids of the genetic code can act, in cognate sequence contexts, as destabilizing N-terminal residues. Advantages of the proposed terms include their brevity and semantic uniformity for N-degrons and C-degrons. In addition to being topologically analogous, N-degrons and C-degrons are related functionally. A proteolytic cleavage of a subunit in a multisubunit complex can create, at the same time, an N-degron (in a C-terminal fragment) and a spatially adjacent C-degron (in an N-terminal fragment). Consequently, both fragments of a subunit can be selectively destroyed through attacks by the N-degron and C-degron pathways.
Keywords: N-end rule; degron; proteasome; proteolysis; ubiquitin.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Johnson ES, Gonda DK, Varshavsky A. Cis-trans recognition and subunit-specific degradation of short-lived proteins. Nature. 1990;346:287–291. - PubMed
-
- Hershko A, Ciechanover A, Varshavsky A. The ubiquitin system. Nat Med. 2000;6:1073–1081. - PubMed
-
- Varshavsky A. Discovery of the biology of the ubiquitin system. JAMA. 2014;311:1969–1970. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

