Belinostat, at Its Clinically Relevant Concentrations, Inhibits Rifampicin-Induced CYP3A4 and MDR1 Gene Expression
- PMID: 30622215
- PMCID: PMC6362450
- DOI: 10.1124/mol.118.114587
Belinostat, at Its Clinically Relevant Concentrations, Inhibits Rifampicin-Induced CYP3A4 and MDR1 Gene Expression
Abstract
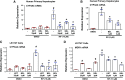
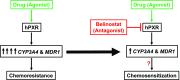
Activation of human pregnane X receptor (hPXR) has been associated with induction of chemoresistance. It has been proposed that such chemoresistance via cytochrome P450/drug transporters can be reversed with the use of antagonists that specifically abrogate agonist-mediated hPXR activation. Unfortunately, proposed antagonists lack the specificity and appropriate pharmacological characteristics that allow these features to be active in the clinic. We propose that, ideally, an hPXR antagonist would be a cancer drug itself that is part of a "cancer drug cocktail" and effective as an hPXR antagonist at therapeutic concentrations. Belinostat (BEL), a histone deacetylase inhibitor approved for the treatment of relapsed/refractory peripheral T-cell lymphoma, and often used in combination with chemotherapy, is an attractive candidate based on its hPXR ligand-like features. We sought to determine whether these features of BEL might allow it to behave as an antagonist in combination chemotherapy regimens that include hPXR activators. BEL represses agonist-activated hPXR target gene expression at its therapeutic concentrations in human primary hepatocytes and LS174T human colon cancer cells. BEL repressed rifampicin-induced gene expression of CYP3A4 and multidrug resistance protein 1, as well as their respective protein activities. BEL decreased rifampicin-induced resistance to SN-38, the active metabolite of irinotecan, in LS174T cells. This finding indicates that BEL could suppress hPXR agonist-induced chemoresistance. BEL attenuated the agonist-induced steroid receptor coactivator-1 interaction with hPXR, and, together with molecular docking studies, the study suggests that BEL directly interacts with multiple sites on hPXR. Taken together, our results suggest that BEL, at its clinically relevant therapeutic concentration, can antagonize hPXR agonist-induced gene expression and chemoresistance.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures





References
-
- Bailey H, McPherson JP, Bailey EB, Werner TL, Gupta S, Batten J, Reddy G, Bhat G, Sharma S, Agarwal N. (2016) A phase I study to determine the pharmacokinetics and urinary excretion of belinostat and metabolites in patients with advanced solid tumors. Cancer Chemother Pharmacol 78:1059–1071. - PubMed
-
- Calvo E, Reddy G, Boni V, García-Cañamaque L, Song T, Tjornelund J, Choi MR, Allen LF. (2016) Pharmacokinetics, metabolism, and excretion of (14)C-labeled belinostat in patients with recurrent or progressive malignancies. Invest New Drugs 34:193–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

