Molecular mechanisms in the pathogenesis of N-nitrosodimethylamine induced hepatic fibrosis
- PMID: 30622238
- PMCID: PMC6325159
- DOI: 10.1038/s41419-018-1272-8
Molecular mechanisms in the pathogenesis of N-nitrosodimethylamine induced hepatic fibrosis
Abstract
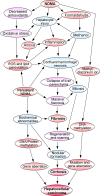
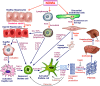
Hepatic fibrosis is marked by excessive synthesis and deposition of connective tissue proteins, especially interstitial collagens in the extracellular matrix of the liver. It is a result of an abnormal wound healing in response to chronic liver injury from various causes such as ethanol, viruses, toxins, drugs, or cholestasis. The chronic stimuli involved in the initiation of fibrosis leads to oxidative stress and generation of reactive oxygen species that serve as mediators of molecular events involved in the pathogenesis of hepatic fibrosis. These processes lead to cellular injury and initiate inflammatory responses releasing a variety of cytokines and growth factors that trigger activation and transformation of resting hepatic stellate cells into myofibroblast like cells, which in turn start excessive synthesis of connective tissue proteins, especially collagens. Uncontrolled and extensive fibrosis results in distortion of lobular architecture of the liver leading to nodular formation and cirrhosis. The perpetual injury and regeneration process could also results in genomic aberrations and mutations that lead to the development of hepatocellular carcinoma. This review covers most aspects of the molecular mechanisms involved in the pathogenesis of hepatic fibrosis with special emphasize on N-Nitrosodimethylamine (NDMA; Dimethylnitorsmaine, DMN) as the inducing agent.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Parola, M. & Pinzani, M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med.S0098-2997, 30070-0 (2018) 10.1016/j.mam.2018.09.002. - PubMed
-
- George J, Rao KR, Stern R, Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129–138. - PubMed
-
- George J, Chandrakasan G. Collagen metabolism in dimethylnitrosamine induced hepatic fibrosis in rats. FASEB J. 1997;11:A1094. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

