Calcium signalling in T cells
- PMID: 30622345
- PMCID: PMC6788797
- DOI: 10.1038/s41577-018-0110-7
Calcium signalling in T cells
Abstract
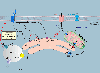
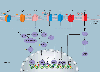
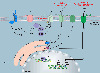
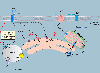
Calcium (Ca2+) signalling is of paramount importance to immunity. Regulated increases in cytosolic and organellar Ca2+ concentrations in lymphocytes control complex and crucial effector functions such as metabolism, proliferation, differentiation, antibody and cytokine secretion and cytotoxicity. Altered Ca2+ regulation in lymphocytes leads to various autoimmune, inflammatory and immunodeficiency syndromes. Several types of plasma membrane and organellar Ca2+-permeable channels are functional in T cells. They contribute highly localized spatial and temporal Ca2+ microdomains that are required for achieving functional specificity. While the mechanistic details of these Ca2+ microdomains are only beginning to emerge, it is evident that through crosstalk, synergy and feedback mechanisms, they fine-tune T cell signalling to match complex immune responses. In this article, we review the expression and function of various Ca2+-permeable channels in the plasma membrane, endoplasmic reticulum, mitochondria and endolysosomes of T cells and their role in shaping immunity and the pathogenesis of immune-mediated diseases.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

