Fractalkine/CX3CR1 Contributes to Endometriosis-Induced Neuropathic Pain and Mechanical Hypersensitivity in Rats
- PMID: 30622457
- PMCID: PMC6309014
- DOI: 10.3389/fncel.2018.00495
Fractalkine/CX3CR1 Contributes to Endometriosis-Induced Neuropathic Pain and Mechanical Hypersensitivity in Rats
Abstract
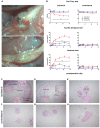
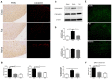
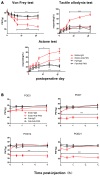
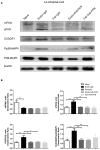
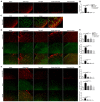
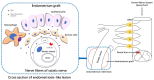
Pain is the most severe and common symptom of endometriosis. Its underlying pathogenetic mechanism is poorly understood. Nerve sensitization is a particular research challenge, due to the limitations of general endometriosis models and sampling nerve tissue from patients. The chemokine fractalkine (FKN) has been demonstrated to play a key role in various forms of neuropathic pain, while its role in endometriotic pain is unknown. Our study was designed to explore the function of FKN in the development and maintenance of peripheral hyperalgesia and central sensitization in endometriosis using a novel endometriosis animal model developed in our laboratory. After modeling, behavioral tests were carried out and the optimal time for molecular changes was obtained. We extracted ectopic tissues and L4-6 spinal cords to detect peripheral and central roles for FKN, respectively. To assess morphologic characteristics of endometriosis-like lesions-as well as expression and location of FKN/CX3CR1-we performed H&E staining, immunostaining, and western blotting analyses. Furthermore, inhibition of FKN expression in the spinal cord was achieved by intrathecal administration of an FKN-neutralizing antibody to demonstrate its function. Our results showed that implanted autologous uterine tissue around the sciatic nerve induced endometriosis-like lesions and produced mechanical hyperalgesia and allodynia. FKN was highly expressed on macrophages, whereas its receptor CX3CR1 was overexpressed in the myelin sheath of sciatic nerve fibers. Overexpressed FKN was also observed in neurons. CX3CR1/pp38-MAPK was upregulated in activated microglia in the spinal dorsal horn. Intrathecal administration of FKN-neutralizing antibody not only reversed the established mechanical hyperalgesia and allodynia, but also inhibited the expression of CX3CR1/pp38-MAPK in activated microglia, which was essential for the persistence of central sensitization. We concluded that the FKN/CX3CR1 signaling pathway might be one of the mechanisms of peripheral hyperalgesia in endometriosis, which requires further studies. Spinal FKN is important for the development and maintenance of central sensitization in endometriosis, and it may further serve as a novel therapeutic target to relieve persistent pain associated with endometriosis.
Keywords: central sensitization; endometriosis; fractalkine; inflammation; microglia; neuropathic pain; peripheral hyperalgesia.
Figures


Similar articles
-
Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice.J Neurochem. 2010 Aug;114(4):1143-57. doi: 10.1111/j.1471-4159.2010.06837.x. Epub 2010 May 28. J Neurochem. 2010. PMID: 20524966
-
Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine.Brain Behav Immun. 2007 Jul;21(5):642-51. doi: 10.1016/j.bbi.2006.11.003. Epub 2006 Dec 15. Brain Behav Immun. 2007. PMID: 17174525 Free PMC article.
-
Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain.Proc Natl Acad Sci U S A. 2007 Jun 19;104(25):10655-60. doi: 10.1073/pnas.0610811104. Epub 2007 Jun 5. Proc Natl Acad Sci U S A. 2007. PMID: 17551020 Free PMC article.
-
Fractalkine/CX3CR1 signalling in chronic pain and inflammation.Curr Pharm Biotechnol. 2011 Oct;12(10):1707-14. doi: 10.2174/138920111798357465. Curr Pharm Biotechnol. 2011. PMID: 21466443 Review.
-
Fractalkine/CX3CR1 signaling during neuropathic pain.Front Cell Neurosci. 2014 May 7;8:121. doi: 10.3389/fncel.2014.00121. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24847207 Free PMC article. Review.
Cited by
-
Identification of miR-30c-5p microRNA in Serum as a Candidate Biomarker to Diagnose Endometriosis.Int J Mol Sci. 2024 Feb 3;25(3):1853. doi: 10.3390/ijms25031853. Int J Mol Sci. 2024. PMID: 38339132 Free PMC article.
-
Role of Macrophages and Mast Cells as Key Players in the Maintenance of Gastrointestinal Smooth Muscle Homeostasis and Disease.Cell Mol Gastroenterol Hepatol. 2022;13(6):1849-1862. doi: 10.1016/j.jcmgh.2022.02.017. Epub 2022 Mar 2. Cell Mol Gastroenterol Hepatol. 2022. PMID: 35245688 Free PMC article. Review.
-
ABX-1431 inhibits the development of endometrial adenocarcinoma and reverses progesterone resistance by targeting MGLL.Cell Death Dis. 2022 Dec 23;13(12):1067. doi: 10.1038/s41419-022-05507-z. Cell Death Dis. 2022. PMID: 36550099 Free PMC article.
-
Contribution of CD4+ cells in the emotional alterations induced by endometriosis in mice.Front Behav Neurosci. 2022 Oct 12;16:946975. doi: 10.3389/fnbeh.2022.946975. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36311856 Free PMC article.
-
Orthopedic surgery-induced cognitive dysfunction is mediated by CX3CL1/R1 signaling.J Neuroinflammation. 2021 Apr 15;18(1):93. doi: 10.1186/s12974-021-02150-x. J Neuroinflammation. 2021. PMID: 33858422 Free PMC article.
References
-
- Arosh J. A., Lee J., Balasubbramanian D., Stanley J. A., Long C. R., Meagher M. W., et al. . (2015). Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc. Natl. Acad. Sci. U S A 112, 9716–9721. 10.1073/pnas.1507931112 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

