Mesenchymal Stromal Cell-Derived Exosomes Affect mRNA Expression and Function of B-Lymphocytes
- PMID: 30622539
- PMCID: PMC6308164
- DOI: 10.3389/fimmu.2018.03053
Mesenchymal Stromal Cell-Derived Exosomes Affect mRNA Expression and Function of B-Lymphocytes
Abstract
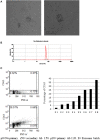
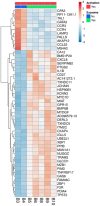
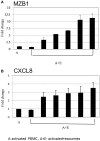
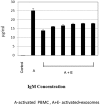
Background: Bone marrow mesenchymal stem cells (bmMSC) may play a role in the regulation of maturation, proliferation, and functional activation of lymphocytes, though the exact mechanisms are unknown. MSC-derived exosomes induce a regulatory response in the function of B, T, and monocyte-derived dendritic cells. Here, we evaluated the specific inhibition of human lymphocytes by bmMSC-derived exosomes and the effects on B-cell function. Methods: Exosomes were isolated from culture media of bmMSC obtained from several healthy donors. The effect of purified bmMSC-derived exosomes on activated peripheral blood mononuclear cells (PBMCs) and isolated B and T lymphocyte proliferation was measured by carboxyfluorescein succinimidyl ester assay. Using the Illumina sequencing platform, mRNA profiling was performed on B-lymphocytes activated in the presence or absence of exosomes. Ingenuity® pathway analysis software was applied to analyze pathway networks, and biological functions of the differentially expressed genes. Validation by RT-PCR was performed. The effect of bmMSC-derived exosomes on antibody secretion was measured by ELISA. Results: Proliferation of activated PBMCs or isolated T and B cells co-cultured with MSC-derived exosomes decreased by 37, 23, and 18%, respectively, compared to controls. mRNA profiling of activated B-lymphocytes revealed 186 genes that were differentially expressed between exosome-treated and control cells. We observed down- and up-regulation of genes that are involved in cell trafficking, development, hemostasis, and immune cell function. RNA-Seq results were validated by real time PCR analysis for the expression of CXCL8 (IL8) and MZB1 genes that are known to have an important role in immune modulation. Functional alterations were confirmed by decreased IgM production levels. Consistent results were demonstrated among a wide variety of healthy human bmMSC donors. Conclusion: Our data show that exosomes may play an important role in immune regulation. They inhibit proliferation of several types of immune cells. In B-lymphocytes they modulate cell function by exerting differential expression of the mRNA of relevant genes. The results of this study help elucidate the mechanisms by which exosomes induce immune regulation and may contribute to the development of newer and safer therapeutic strategies.
Keywords: B-lymphocytes; CXCL8; MZB1; bmMSC-derived exosomes; ingenuity pathway analysis; mesenchymal stem cells; next generation sequencing.
Figures


References
-
- Gurevitch O, Slavin S, Resnick I, Khitrin S, Feldman A. Mesenchymal progenitor cells in red and yellow bone marrow. Folia Biol. (2009) 55:27–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

