Mesenchymal Stem Cells Ameliorate Hepatic Ischemia/Reperfusion Injury via Inhibition of Neutrophil Recruitment
- PMID: 30622980
- PMCID: PMC6304871
- DOI: 10.1155/2018/7283703
Mesenchymal Stem Cells Ameliorate Hepatic Ischemia/Reperfusion Injury via Inhibition of Neutrophil Recruitment
Abstract
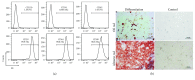
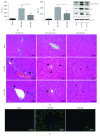
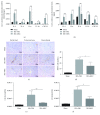
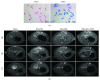
Ischemia/reperfusion injury (IRI) remains a major problem in organ transplantation, which represents the main cause of graft dysfunction posttransplantation. Hepatic IRI is characterized by an excessive inflammatory response within the liver. Mesenchymal stem cells (MSCs) have been shown to be immunomodulatory cells and have the therapeutic action on IRI in several organs. However, the mechanism of regulatory effect of MSCs on IRI remains unclear. In the present study, we examined the impact of MSCs on hepatic inflammatory response such as neutrophil influx and liver damage in a rat model of 70% hepatic IRI. Treatment with MSCs protected rat against hepatic IRI, with significantly decreased serum levels of liver enzymes, attenuated hepatic neutrophil infiltration, reduced expression of apoptosis-associated proteins, and ameliorated liver pathological injury. MSCs also significantly enhanced the intracellular activation of p38 MAPK phosphorylation, which led to decreased expression of CXCR2 on the surface of neutrophils. In addition, MSCs significantly diminished neutrophil chemoattractant CXCL2 production by inhibiting NF-κB p65 phosphorylation in macrophages. These results demonstrate that MSCs significantly ameliorate hepatic IRI predominantly through its inhibitory effect on hepatic neutrophil migration and infiltration.
Figures
References
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

