Induction of functional islet-like cells from human iPS cells by suspension culture
- PMID: 30623004
- PMCID: PMC6317273
- DOI: 10.1016/j.reth.2018.11.003
Induction of functional islet-like cells from human iPS cells by suspension culture
Abstract
Introduction: To complement islet transplantation for type1 diabetic patients, cell-based therapy using pluripotent stem cells such as ES cells and iPS cells is promising. Many papers have already reported the induction of pancreatic β cells from these cell types, but a suspension culture system has not usually been employed. The aim of this study is to establish a suspension culture method for inducing functional islet-like cells from human iPS cells.
Methods: We used 30 ml spinner type culture vessels for human iPS cells throughout the differentiation process. Differentiated cells were analyzed by immunostaining and C-peptide secretion. Cell transplantation experiments were performed with STZ-induced diabetic NOD/SCID mice. Blood human C-peptide and glucagon levels were measured serially in mice, and grafts were analyzed histologically.
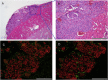
Results: We obtained spherical pancreatic beta-like cells from human iPS cells and detected verifiable amounts of C-peptide secretion in vitro. We demonstrated reversal of hyperglycemia in diabetic model mice after transplantation of these cells, maintaining non-fasting blood glucose levels along with the human glycemic set point. We confirmed the secretion of human insulin and glucagon dependent on the blood glucose level in vivo. Immunohistological analysis revealed that grafted cells became α, β and δ cells in vivo.
Conclusions: These results suggest that differentiated cells derived from human iPS cells grown in suspension culture mature and function like pancreatic islets in vivo.
Keywords: Islet; Pancreatic β cell; iPS cells.
Figures




References
-
- Melton D.A. Applied developmental biology: making human pancreatic beta cells for diabetics. Curr Top Dev Biol. 2016;117:65–73. - PubMed
-
- Pellegrini S., Cantarelli E., Sordi V., Nano R., Piemonti L. The state of the art of islet transplantation and cell therapy in type 1 diabetes. Acta Diabetol. 2016;53:683–691. - PubMed
-
- D'Amour K.A., Bang A.G., Eliazer S., Kelly O.G., Agulnick A.D., Smart N.G. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

