Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy
- PMID: 30623136
- PMCID: PMC6314973
- DOI: 10.1016/j.jacbts.2018.08.006
Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy
Abstract
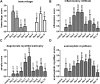
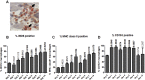
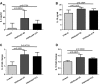
Takotsubo cardiomyopathy is an acute stress-induced heart failure syndrome for which the exact pathogenic mechanisms are unclear, and consequently, no specific treatment exists. In an experimental model of stress-induced takotsubo-like cardiomyopathy, the authors describe the temporal course of a chronic inflammatory response post-induction, with an initial early influx of neutrophils into myocardial tissue followed by macrophages that are typical of a proinflammatory M1 phenotype, and a nonsignificant increase in systemic inflammatory cytokines. Post-mortem myocardium from the more complex clinical takotsubo patients share features of the study's experimental model. These findings suggest modulators of inflammation could be a potential therapeutic option.
Keywords: EF, ejection fraction; IL, interleukin; MHC, major histocompatibility complex; MI, myocardial infarction; TNFα, tumor necrosis factor-alpha; histopathology; inflammation; macrophage; pathophysiology; qPCR, quantitative polymerase chain reaction; takotsubo cardiomyopathy.
Figures




References
-
- Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. - PubMed
-
- Tornvall P., Collste O., Ehrenborg E., Jarnbert-Petterson H. A case-control study of risk markers and mortality in takotsubo stress cardiomyopathy. J Am Coll Cardiol. 2016;67:1931–1936. - PubMed
-
- Stiermaier T., Moeller C., Oehler K. Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail. 2016;18:650–656. - PubMed
-
- Dawson D.K., Neil C.J., Henning A. Tako-Tsubo cardiomyopathy: a heart stressed out of energy? J Am Coll Cardiol Img. 2015;8:985–987. - PubMed
-
- Scally C., Ahearn T., Rudd A. Right ventricular involvement and recovery after acute stress-induced (tako-tsubo) cardiomyopathy. Am J Cardiol. 2016;117:775–780. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

