Metabolic engineering of bread wheat improves grain iron concentration and bioavailability
- PMID: 30623558
- PMCID: PMC6662306
- DOI: 10.1111/pbi.13074
Metabolic engineering of bread wheat improves grain iron concentration and bioavailability
Abstract
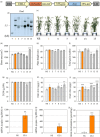
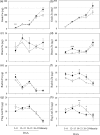
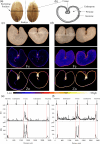
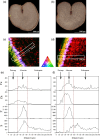
Bread wheat (Triticum aestivum L.) is cultivated on more land than any other crop and produces a fifth of the calories consumed by humans. Wheat endosperm is rich in starch yet contains low concentrations of dietary iron (Fe) and zinc (Zn). Biofortification is a micronutrient intervention aimed at increasing the density and bioavailability of essential vitamins and minerals in staple crops; Fe biofortification of wheat has proved challenging. In this study we employed constitutive expression (CE) of the rice (Oryza sativa L.) nicotianamine synthase 2 (OsNAS2) gene in bread wheat to up-regulate biosynthesis of two low molecular weight metal chelators - nicotianamine (NA) and 2'-deoxymugineic acid (DMA) - that play key roles in metal transport and nutrition. The CE-OsNAS2 plants accumulated higher concentrations of grain Fe, Zn, NA and DMA and synchrotron X-ray fluorescence microscopy (XFM) revealed enhanced localization of Fe and Zn in endosperm and crease tissues, respectively. Iron bioavailability was increased in white flour milled from field-grown CE-OsNAS2 grain and positively correlated with NA and DMA concentrations.
Keywords: 2′-deoxymugineic acid; Caco-2; X-ray fluorescence microscopy; Zinc; biofortification; nicotianamine.
© 2019 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Berger, B. , De Regt, B. and Tester, M. (2012) High‐throughput phenotyping in plants. Methods Mol. Biol. 918, 9–20. - PubMed
-
- Bouis, H.E. , Hotz, C. , McClafferty, B. , Meenakshi, J.V. and Pfeiffer, W.H. (2011) Biofortification: a new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 32, S31–S40. - PubMed
-
- Brouns, F. , Hemery, Y. , Price, R. and Anson, N.M. (2012) Wheat aleurone: separation, composition, health aspects, and potential food use. Crit. Rev. Food Sci. Nutr. 52, 553–568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

