Computational Modeling of Realistic Cell Membranes
- PMID: 30623647
- PMCID: PMC6509646
- DOI: 10.1021/acs.chemrev.8b00460
Computational Modeling of Realistic Cell Membranes
Abstract
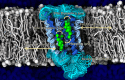
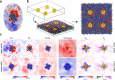
Cell membranes contain a large variety of lipid types and are crowded with proteins, endowing them with the plasticity needed to fulfill their key roles in cell functioning. The compositional complexity of cellular membranes gives rise to a heterogeneous lateral organization, which is still poorly understood. Computational models, in particular molecular dynamics simulations and related techniques, have provided important insight into the organizational principles of cell membranes over the past decades. Now, we are witnessing a transition from simulations of simpler membrane models to multicomponent systems, culminating in realistic models of an increasing variety of cell types and organelles. Here, we review the state of the art in the field of realistic membrane simulations and discuss the current limitations and challenges ahead.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

