Synthesis and O-Glycosidic Linkage Conformational Analysis of 13C-Labeled Oligosaccharide Fragments of an Antifreeze Glycolipid
- PMID: 30624062
- PMCID: PMC8224223
- DOI: 10.1021/acs.joc.8b01411
Synthesis and O-Glycosidic Linkage Conformational Analysis of 13C-Labeled Oligosaccharide Fragments of an Antifreeze Glycolipid
Abstract
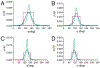
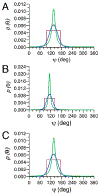
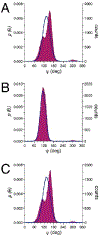
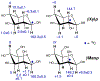
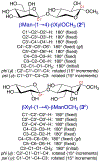
NMR studies of two 13C-labeled disaccharides and a tetrasaccharide were undertaken that comprise the backbone of a novel thermal hysteresis glycolipid containing a linear glycan sequence of alternating [βXyl p-(1→4)-βMan p-(1→4)] n dimers. Experimental trans-glycoside NMR J-couplings, parameterized equations obtained from density functional theory (DFT) calculations, and an in-house circular statistics package ( MA'AT) were used to derive conformational models of linkage torsion angles ϕ and ψ in solution, which were compared to those obtained from molecular dynamics simulations. Modeling using different probability distribution functions showed that MA'AT models of ϕ in βMan(1→4)βXyl and βXyl(1→4)βMan linkages are very similar in the disaccharide building blocks, whereas MA'AT models of ψ differ. This pattern is conserved in the tetrasaccharide, showing that linkage context does not influence linkage geometry in this linear system. Good agreement was observed between the MA'AT and MD models of ψ with respect to mean values and circular standard deviations. Significant differences were observed for ϕ, indicating that revision of the force-field employed by GLYCAM is probably needed. Incorporation of the experimental models of ϕ and ψ into the backbone of an octasaccharide fragment leads to a helical amphipathic topography that may affect the thermal hysteresis properties of the glycolipid.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




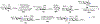



References
-
- Lemieux R; Koto S The Conformational Properties of Glycosidic Linkages. Tetrahedron 1974, 30, 1933–1944.
-
- Landersjö C; Stenutz R; Widmalm G Conformational Flexibility of Carbohydrates: A Folded Conformer at the ϕ Dihedral Angle of a Glycosidic Linkage. J. Am. Chem. Soc 1997, 119, 8695–8698.
-
- Thøgersen H; Lemieux RU; Bock K; Meyer B Further Justification for the Exo- Anomeric Effect. Conformational Analysis Based on Nuclear Magnetic Resonance Spectroscopy of Oligosaccharides. Can. J. Chem 1982, 60, 44–57.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

