Chemical Analysis of the Interface between Hybrid Organic-Inorganic Perovskite and Atomic Layer Deposited Al2O3
- PMID: 30624886
- PMCID: PMC6369720
- DOI: 10.1021/acsami.8b18307
Chemical Analysis of the Interface between Hybrid Organic-Inorganic Perovskite and Atomic Layer Deposited Al2O3
Abstract
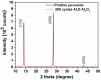
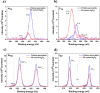
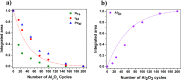
Ultrathin metal oxides prepared by atomic layer deposition (ALD) have gained utmost attention as moisture and thermal stress barrier layers in perovskite solar cells (PSCs). We have recently shown that 10 cycles of ALD Al2O3 deposited directly on top of the CH3NH3PbI3- xCl x perovskite material, are effective in delivering a superior PSC performance with 18% efficiency (compared to 15% of the Al2O3-free cell) with a long-term humidity-stability of more than 60 days. Motivated by these results, the present contribution focuses on the chemical modification which the CH3NH3PbI3- xCl x perovskite undergoes upon growth of ALD Al2O3. Specifically, we combine in situ Infrared (IR) spectroscopy studies during film growth, together with X-ray photoelectron spectroscopy (XPS) analysis of the ALD Al2O3/perovskite interface. The IR-active signature of the NH3+ stretching mode of the perovskite undergoes minimal changes upon exposure to ALD cycles, suggesting no diffusion of ALD precursor and co-reactant (Al(CH3)3 and H2O) into the bulk of the perovskite. However, by analyzing the difference between the IR spectra associated with the Al2O3 coated perovskite and the pristine perovskite, respectively, changes occurring at the surface of perovskite are monitored. The abstraction of either NH3 or CH3NH2 from the perovskite surface is observed as deduced by the development of negative N-H bands associated with its stretching and bending modes. The IR investigations are corroborated by XPS study, confirming the abstraction of CH3NH2 from the perovskite surface, whereas no oxidation of its inorganic framework is observed within the ALD window process investigated in this work. In parallel, the growth of ALD Al2O3 on perovskite is witnessed by the appearance of characteristic IR-active Al-O-Al phonon and (OH)-Al═O stretching modes. Based on the IR and XPS investigations, a plausible growth mechanism of ALD Al2O3 on top of perovskite is presented.
Keywords: Al2O3; X-ray photoelectron spectroscopy; atomic layer deposition; infrared spectroscopy; perovskite.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- NREL. Best Research-Cell Efficiencies http://www.nrel.gov/ncpv/images/efficiency_chart.jpg (accessed September 13, 2018).
-
- Wang D.; Wright M.; Elumalai N. K.; Uddin A. Stability of Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. 10.1016/j.solmat.2015.12.025. - DOI
-
- Niu G.; Guo X.; Wang L. Review of Recent Progress in Chemical Stability of Perovskite Solar Cells. J. Mater. Chem. A 2015, 3 (17), 8970–8980. 10.1039/C4TA04994B. - DOI
-
- Conings B.; Drijkoningen J.; Gauquelin N.; Babayigit A.; D’Haen J.; D’Olieslaeger L.; Ethirajan A.; Verbeeck J.; Manca J.; Mosconi E.; Angelis F. D.; Boyen H.-G. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5 (15), 1500477.10.1002/aenm.201500477. - DOI
LinkOut - more resources
Full Text Sources

