Mechanistic Landscape of Membrane-Permeabilizing Peptides
- PMID: 30624911
- PMCID: PMC9235363
- DOI: 10.1021/acs.chemrev.8b00520
Mechanistic Landscape of Membrane-Permeabilizing Peptides
Abstract
Membrane permeabilizing peptides (MPPs) are as ubiquitous as the lipid bilayer membranes they act upon. Produced by all forms of life, most membrane permeabilizing peptides are used offensively or defensively against the membranes of other organisms. Just as nature has found many uses for them, translational scientists have worked for decades to design or optimize membrane permeabilizing peptides for applications in the laboratory and in the clinic ranging from antibacterial and antiviral therapy and prophylaxis to anticancer therapeutics and drug delivery. Here, we review the field of membrane permeabilizing peptides. We discuss the diversity of their sources and structures, the systems and methods used to measure their activities, and the behaviors that are observed. We discuss the fact that "mechanism" is not a discrete or a static entity for an MPP but rather the result of a heterogeneous and dynamic ensemble of structural states that vary in response to many different experimental conditions. This has led to an almost complete lack of discrete three-dimensional active structures among the thousands of known MPPs and a lack of useful or predictive sequence-structure-function relationship rules. Ultimately, we discuss how it may be more useful to think of membrane permeabilizing peptides mechanisms as broad regions of a mechanistic landscape rather than discrete molecular processes.
Figures




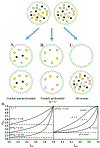

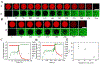

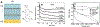







References
-
- Bechinger B Rationalizing the Membrane Interactions of Cationic Amphipathic Antimicrobial Peptides by Their Molecular Shape. Curr. Opin. Colloid Interface Sci 2009, 14, 349–355.
-
- Bechinger B; Lohner K Detergent-like Actions of Linear Amphipathic Cationic Antimicrobial Peptides. Biochim. Biophys. Acta - Biomembr 2006, 1758, 1529–1539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

