Neuronal drebrin A directly interacts with mDia2 formin to inhibit actin assembly
- PMID: 30625038
- PMCID: PMC6589693
- DOI: 10.1091/mbc.E18-10-0639
Neuronal drebrin A directly interacts with mDia2 formin to inhibit actin assembly
Abstract
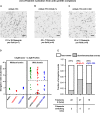
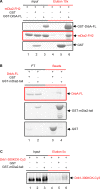
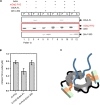
Dendritic spines (DS) are actin-rich postsynaptic terminals of neurons that are critical for higher-order brain functions. Maturation of DS is accompanied by a change in actin architecture from linear to branched filamentous structures. Presumably, the underlying cause of this is a switch in a mode of actin assembly from formin-driven to Arp2/3-mediated via an undefined mechanism. Here we present data suggesting that neuron-specific actin-binding drebrin A may be a part of such a switch. It is well documented that DS are highly enriched in drebrin A, which is critical for their plasticity and function. At the same time, mDia2 is known to mediate the formation of filopodia-type (immature) spines. We found that neuronal drebrin A directly interacts with mDia2 formin. Drebrin inhibits formin-mediated nucleation of actin and abolishes mDia2-induced actin bundling. Using truncated protein constructs we identified the domain requirements for drebrin-mDia2 interaction. We hypothesize that accumulation of drebrin A in DS (that coincides with spine maturation) leads to inhibition of mDia2-driven actin polymerization and, therefore, may contribute to a change in actin architecture from linear to branched filaments.
Figures



Similar articles
-
Mapping Molecular Interaction Interface Between Diaphanous Formin-2 and Neuron-Specific Drebrin A.J Mol Biol. 2023 Dec 15;435(24):168334. doi: 10.1016/j.jmb.2023.168334. Epub 2023 Oct 26. J Mol Biol. 2023. PMID: 37898384
-
The filamentous actin cross-linking/bundling activity of mammalian formins.J Mol Biol. 2008 Dec 12;384(2):324-34. doi: 10.1016/j.jmb.2008.09.043. Epub 2008 Sep 26. J Mol Biol. 2008. PMID: 18835565
-
Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules.Mol Biol Cell. 2011 Dec;22(23):4575-87. doi: 10.1091/mbc.E11-07-0616. Epub 2011 Oct 12. Mol Biol Cell. 2011. PMID: 21998204 Free PMC article.
-
The role of drebrin in dendritic spines.Mol Cell Neurosci. 2017 Oct;84:85-92. doi: 10.1016/j.mcn.2017.01.004. Epub 2017 Feb 1. Mol Cell Neurosci. 2017. PMID: 28161364 Review.
-
Remodeling of Actin Filaments by Drebrin A and Its Implications.Adv Exp Med Biol. 2017;1006:61-82. doi: 10.1007/978-4-431-56550-5_5. Adv Exp Med Biol. 2017. PMID: 28865015 Review.
Cited by
-
ARHGEF5 binds Drebrin and affects α-tubulin acetylation to direct neuronal morphogenesis and migration during mouse brain development.Front Mol Neurosci. 2024 Jun 12;17:1421932. doi: 10.3389/fnmol.2024.1421932. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38932934 Free PMC article.
-
Nuclear protein FNBP4: A novel inhibitor of non-diaphanous formin FMN1-mediated actin cytoskeleton dynamics.J Biol Chem. 2025 Jun;301(6):108550. doi: 10.1016/j.jbc.2025.108550. Epub 2025 Apr 30. J Biol Chem. 2025. PMID: 40316024 Free PMC article.
-
Profilin and Mical combine to impair F-actin assembly and promote disassembly and remodeling.Nat Commun. 2021 Sep 20;12(1):5542. doi: 10.1038/s41467-021-25781-3. Nat Commun. 2021. PMID: 34545088 Free PMC article.
-
Drebrin controls scar formation and astrocyte reactivity upon traumatic brain injury by regulating membrane trafficking.Nat Commun. 2021 Mar 5;12(1):1490. doi: 10.1038/s41467-021-21662-x. Nat Commun. 2021. PMID: 33674568 Free PMC article.
-
A framework to validate fluorescently labeled DNA-binding proteins for single-molecule experiments.Cell Rep Methods. 2023 Oct 23;3(10):100614. doi: 10.1016/j.crmeth.2023.100614. Epub 2023 Oct 12. Cell Rep Methods. 2023. PMID: 37832544 Free PMC article.
References
-
- Alberts AS. (2001). Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem , 2824–2830. - PubMed
-
- Almuqbil M, Hamdan FF, Mathonnet G, Rosenblatt B, Srour M. (2013). De novo deletion of FMN2 in a girl with mild non-syndromic intellectual disability. Eur J Med Genet , 686–688. - PubMed
-
- Bertling E, Hotulainen P. (2017). New waves in dendritic spine actin cytoskeleton: from branches and bundles to rings, from actin binding proteins to post-translational modifications. Mol Cell Neurosci , 77–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

