Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms
- PMID: 30625189
- PMCID: PMC6326569
- DOI: 10.1371/journal.pone.0210064
Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms
Abstract
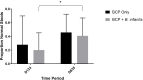
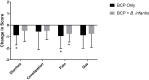
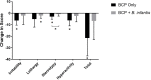
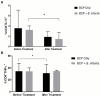
Over half of all children with autism spectrum disorders (ASD) have gastrointestinal (GI) co-morbidities including chronic constipation, diarrhea, and irritable bowel syndrome. The severity of these symptoms has been correlated with the degree of GI microbial dysbiosis. The study objective was to assess tolerability of a probiotic (Bifidobacterium infantis) in combination with a bovine colostrum product (BCP) as a source of prebiotic oligosaccharides and to evaluate GI, microbiome and immune factors in children with ASD and GI co-morbidities. This pilot study is a randomized, double blind, controlled trial of combination treatment (BCP + B. infantis) vs. BCP alone in a cross-over study in children ages 2-11 with ASD and GI co-morbidities (n = 8). This 12-week study included 5 weeks of probiotic-prebiotic supplementation, followed by a two-week washout period, and 5 weeks of prebiotic only supplementation. The primary outcome of tolerability was assessed using validated questionnaires of GI function and atypical behaviors, along with side effects. Results suggest that the combination treatment is well-tolerated in this cohort. The most common side effect was mild gassiness. Some participants on both treatments saw a reduction in the frequency of certain GI symptoms, as well as reduced occurrence of particular aberrant behaviors. Improvement may be explained by a reduction in IL-13 and TNF-α production in some participants. Although limited conclusions can be drawn from this small pilot study, the results support the need for further research into the efficacy of these treatments.
Conflict of interest statement
J. Bruce German and David A Mills are co-founders of Evolve Biosystems, a company focused on diet-based manipulation of the gut microbiota. Evolve Biosystems played no role in the design, execution, interpretation, or publication of this study. This conflict does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures




References
-
- Kral TV, Eriksen WT, Souders MC, Pinto-Martin JA. Eating Behaviors, Diet Quality, and Gastrointestinal Symptoms in Children With Autism Spectrum Disorders: A Brief Review. Journal of pediatric nursing. 2013. - PubMed
-
- de Theije CG, Wu J, da Silva SL, Kamphuis PJ, Garssen J, Korte SM, et al. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. European journal of pharmacology. 2011;668 Suppl 1:S70–80. - PubMed
-
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Applied and environmental microbiology. 2011;77(18):6718–21. 10.1128/AEM.05212-11 - DOI - PMC - PubMed

