Cytosolic Isocitrate Dehydrogenase from Arabidopsis thaliana Is Regulated by Glutathionylation
- PMID: 30625997
- PMCID: PMC6356969
- DOI: 10.3390/antiox8010016
Cytosolic Isocitrate Dehydrogenase from Arabidopsis thaliana Is Regulated by Glutathionylation
Abstract
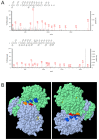
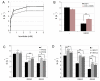
NADP-dependent (Nicotinamide Adénine Dinucléotide Phosphate-dependent) isocitrate dehydrogenases (NADP-ICDH) are metabolic enzymes involved in 2-oxoglutarate biosynthesis, but they also supply cells with NADPH. Different NADP-ICDH genes are found in Arabidopsis among which a single gene encodes for a cytosolic ICDH (cICDH) isoform. Here, we show that cICDH is susceptible to oxidation and that several cysteine (Cys) residues are prone to S-nitrosylation upon nitrosoglutathione (GSNO) treatment. Moreover, we identified a single S-glutathionylated cysteine Cys363 by mass-spectrometry analyses. Modeling analyses suggest that Cys363 is not located in the close proximity of the cICDH active site. In addition, mutation of Cys363 consistently does not modify the activity of cICDH. However, it does affect the sensitivity of the enzyme to GSNO, indicating that S-glutathionylation of Cys363 is involved in the inhibition of cICDH activity upon GSNO treatments. We also show that glutaredoxin are able to rescue the GSNO-dependent inhibition of cICDH activity, suggesting that they act as a deglutathionylation system in vitro. The glutaredoxin system, conversely to the thioredoxin system, is able to remove S-nitrosothiol adducts from cICDH. Finally, NADP-ICDH activities were decreased both in a catalase2 mutant and in mutants affected in thiol reduction systems, suggesting a role of the thiol reduction systems to protect NADP-ICDH activities in planta. In line with our observations in Arabidopsis, we found that the human recombinant NADP-ICDH activity is also sensitive to oxidation in vitro, suggesting that this redox mechanism might be shared by other ICDH isoforms.
Keywords: Arabidopsis thaliana; Isocitrate dehydrogenase; glutaredoxin; glutathionylation; nitrosylation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Cytosolic NADP-dependent isocitrate dehydrogenase contributes to redox homeostasis and the regulation of pathogen responses in Arabidopsis leaves.Plant Cell Environ. 2010 Jul;33(7):1112-23. doi: 10.1111/j.1365-3040.2010.02133.x. Epub 2010 Mar 1. Plant Cell Environ. 2010. PMID: 20199623
-
Inactivation of NADP(+)-dependent isocitrate dehydrogenase by nitric oxide.Free Radic Biol Med. 2002 Oct 1;33(7):927-37. doi: 10.1016/s0891-5849(02)00981-4. Free Radic Biol Med. 2002. PMID: 12361803
-
Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite. Implications for cytotoxicity and alcohol-induced liver injury.J Biol Chem. 2003 Dec 19;278(51):51360-71. doi: 10.1074/jbc.M302332200. Epub 2003 Oct 9. J Biol Chem. 2003. PMID: 14551203
-
Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants.J Exp Bot. 2021 Feb 11;72(3):830-847. doi: 10.1093/jxb/eraa440. J Exp Bot. 2021. PMID: 32945878 Review.
-
Control of isocitrate dehydrogenase catalytic activity by protein phosphorylation in Escherichia coli.J Mol Microbiol Biotechnol. 2005;9(3-4):132-46. doi: 10.1159/000089642. J Mol Microbiol Biotechnol. 2005. PMID: 16415587 Review.
Cited by
-
Genetic and transcriptomic dissection of host defense to Goss's bacterial wilt and leaf blight of maize.G3 (Bethesda). 2023 Nov 1;13(11):jkad197. doi: 10.1093/g3journal/jkad197. G3 (Bethesda). 2023. PMID: 37652038 Free PMC article.
-
Central Metabolism in Mammals and Plants as a Hub for Controlling Cell Fate.Antioxid Redox Signal. 2021 May 1;34(13):1025-1047. doi: 10.1089/ars.2020.8121. Epub 2020 Aug 5. Antioxid Redox Signal. 2021. PMID: 32620064 Free PMC article. Review.
-
Thioredoxin and Glutaredoxin Systems Antioxidants Special Issue.Antioxidants (Basel). 2019 Mar 18;8(3):68. doi: 10.3390/antiox8030068. Antioxidants (Basel). 2019. PMID: 30889816 Free PMC article.
-
Post-Translational Modifications to Cysteine Residues in Plant Proteins and Their Impact on the Regulation of Metabolism and Signal Transduction.Int J Mol Sci. 2024 Sep 12;25(18):9845. doi: 10.3390/ijms25189845. Int J Mol Sci. 2024. PMID: 39337338 Free PMC article. Review.
-
Biological Functions of Hydrogen Sulfide in Plants.Int J Mol Sci. 2022 Dec 1;23(23):15107. doi: 10.3390/ijms232315107. Int J Mol Sci. 2022. PMID: 36499443 Free PMC article. Review.
References
-
- Møller I.M., Rasmusson A.G. The role of NADP in the mitochondrial matrix. Trends Plant Sci. 1998;3:21–27.
-
- Kruse A., Fieuw S., Heineke D., Müller-Röber B. Antisens inhibition of cytosolic NADP-dependent isocitrate dehydrogenase in transgenic potato plants. Planta. 1998;205:82–91. doi: 10.1007/s004250050299. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

