Reproductive Senescence in Drones of the Honey Bee (Apis mellifera)
- PMID: 30626026
- PMCID: PMC6358831
- DOI: 10.3390/insects10010011
Reproductive Senescence in Drones of the Honey Bee (Apis mellifera)
Abstract
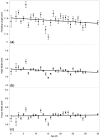
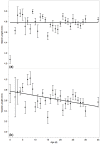
In the face of high proportions of yearly colony losses, queen health and fecundity has been a major focus of industry and research. Much of the reproductive quality of the queen, though, is a function of the mating success and quality of the drones (males). Many environmental factors can negatively impact drone semen quality, but little is known about factors that impact the drones' ability to successfully mate and deliver that semen, or how widely drones vary. In our study, we observed the daily variation in honey bee drone reproductive quality over time, along with a number of morphological traits. Drones were reared in cages in bank colonies, and 20 individuals were dissected and measured daily. The number of viable spermatozoa in the seminal vesicles was zero at emergence and reached an average maximum of 7.39 ± 0.19 million around 20 days of life. Decline in spermatozoa count occurred after day 30, though viability was constant throughout life, when controlling for count. Older drones had smaller wet weights, head widths, and wing lengths. We predict that this is likely due to sampling bias due to a differential lifespan among larger, more reproductively developed drones. Our study shows that drones are more highly variable than previously suggested and that they have a significant variation in reproductive physiology as a function of age.
Keywords: Apis mellifera; aging; drone; honey bee; reproduction; senescence; sperm viability; spermatozoa.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Ellis J.D., Evans J.D., Pettis J. Colony losses, managed colony population decline, and colony collapse disorder in the United States. J. Apic. Res. 2010;49:134–136. doi: 10.3896/IBRA.1.49.1.30. - DOI
-
- Seitz N., Traynor K.S., Steinhauer N., Rennich K., Wilson M.E., Ellis J.D., Rose R., Tarpy D.R., Sagili R.R., Caron D.M., et al. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apic. Res. 2015;54:292–304. doi: 10.1080/00218839.2016.1153294. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

