Modulation of RAB7A Protein Expression Determines Resistance to Cisplatin through Late Endocytic Pathway Impairment and Extracellular Vesicular Secretion
- PMID: 30626032
- PMCID: PMC6357196
- DOI: 10.3390/cancers11010052
Modulation of RAB7A Protein Expression Determines Resistance to Cisplatin through Late Endocytic Pathway Impairment and Extracellular Vesicular Secretion
Abstract
Background: Cisplatin (CDDP) is widely used in treatment of cancer, yet patients often develop resistance with consequent therapeutical failure. In CDDP-resistant cells alterations of endocytosis and lysosomal functionality have been revealed, although their causes and contribution to therapy response are unclear.
Methods: We investigated the role of RAB7A, a key regulator of late endocytic trafficking, in CDDP-resistance by comparing resistant and sensitive cells using western blotting, confocal microscopy and real time PCR. Modulation of RAB7A expression was performed by transfection and RNA interference, while CDDP sensitivity and intracellular accumulation were evaluated by viability assays and chemical approaches, respectively. Also extracellular vesicles were purified and analyzed. Finally, correlations between RAB7A and chemotherapy response was investigated in human patient samples.
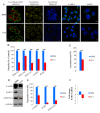
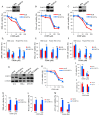
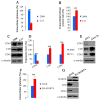
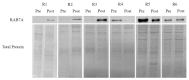
Results: We demonstrated that down-regulation of RAB7A characterizes the chemoresistant phenotype, and that RAB7A depletion increases CDDP-resistance while RAB7A overexpression decreases it. In addition, increased production of extracellular vesicles is modulated by RAB7A expression levels and correlates with reduction of CDDP intracellular accumulation.
Conclusions: We demonstrated, for the first time, that RAB7A regulates CDDP resistance determining alterations in late endocytic trafficking and drug efflux through extracellular vesicles.
Keywords: RAB7A; chemoresistance; cisplatin; endocytosis; lysosome.
Conflict of interest statement
The authors declare that they have no conflicting financial interest.
Figures
Similar articles
-
Defects of mitochondria-lysosomes communication induce secretion of mitochondria-derived vesicles and drive chemoresistance in ovarian cancer cells.Cell Commun Signal. 2024 Mar 6;22(1):165. doi: 10.1186/s12964-024-01507-y. Cell Commun Signal. 2024. PMID: 38448982 Free PMC article.
-
[Effect of down-regulation of survivin gene on apoptosis and cisplatin resistance in cisplatin resistant human lung adenocarcinoma A549/CDDP cells].Zhonghua Zhong Liu Za Zhi. 2006 Jun;28(6):408-12. Zhonghua Zhong Liu Za Zhi. 2006. PMID: 17152483 Chinese.
-
Forkhead Box Protein C2 (FOXC2) Promotes the Resistance of Human Ovarian Cancer Cells to Cisplatin In Vitro and In Vivo.Cell Physiol Biochem. 2016;39(1):242-52. doi: 10.1159/000445620. Epub 2016 Jun 24. Cell Physiol Biochem. 2016. PMID: 27336949
-
Rab7a regulates cell migration through Rac1 and vimentin.Biochim Biophys Acta Mol Cell Res. 2017 Feb;1864(2):367-381. doi: 10.1016/j.bbamcr.2016.11.020. Epub 2016 Nov 23. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27888097
-
Role of the RAB7 Protein in Tumor Progression and Cisplatin Chemoresistance.Cancers (Basel). 2019 Aug 1;11(8):1096. doi: 10.3390/cancers11081096. Cancers (Basel). 2019. PMID: 31374919 Free PMC article. Review.
Cited by
-
DNA Containing Cyclobutane Pyrimidine Dimers Is Released from UVB-Irradiated Keratinocytes in a Caspase-Dependent Manner.J Invest Dermatol. 2022 Nov;142(11):3062-3070.e3. doi: 10.1016/j.jid.2022.04.030. Epub 2022 Jun 9. J Invest Dermatol. 2022. PMID: 35691362 Free PMC article.
-
Mitochondrial-Derived Vesicles: The Good, the Bad, and the Ugly.Int J Mol Sci. 2023 Sep 8;24(18):13835. doi: 10.3390/ijms241813835. Int J Mol Sci. 2023. PMID: 37762138 Free PMC article. Review.
-
Potential Prognostic Role of 18F-FDG PET/CT in Invasive Epithelial Ovarian Cancer Relapse. A Preliminary Study.Cancers (Basel). 2019 May 23;11(5):713. doi: 10.3390/cancers11050713. Cancers (Basel). 2019. PMID: 31126127 Free PMC article.
-
New Insights into Mechanisms of Cisplatin Resistance: From Tumor Cell to Microenvironment.Int J Mol Sci. 2019 Aug 24;20(17):4136. doi: 10.3390/ijms20174136. Int J Mol Sci. 2019. PMID: 31450627 Free PMC article. Review.
-
Role of Circulating miRNAs in Therapeutic Response in Epithelial Ovarian Cancer: A Systematic Revision.Biomedicines. 2021 Sep 26;9(10):1316. doi: 10.3390/biomedicines9101316. Biomedicines. 2021. PMID: 34680433 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

