Short Peptides with Uncleavable Peptide Bond Mimetics as Photoactivatable Caspase-3 Inhibitors
- PMID: 30626051
- PMCID: PMC6337261
- DOI: 10.3390/molecules24010206
Short Peptides with Uncleavable Peptide Bond Mimetics as Photoactivatable Caspase-3 Inhibitors
Abstract
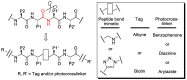
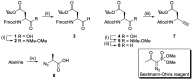
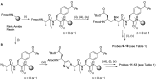
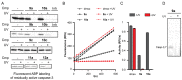
Chemical probes that covalently interact with proteases have found increasing use for the study of protease function and localization. The design and synthesis of such probes is still a bottleneck, as the strategies to target different families are highly diverse. We set out to design and synthesize chemical probes based on protease substrate specificity with inclusion of an uncleavable peptide bond mimic and a photocrosslinker for covalent modification of the protease target. With caspase-3 as a model target protease, we designed reduced amide and triazolo peptides as substrate mimetics, whose sequences can be conveniently constructed by modified solid phase peptide synthesis. We found that these probes inhibited the caspase-3 activity, but did not form a covalent bond. It turned out that the reduced amide mimics, upon irradiation with a benzophenone as photosensitizer, are oxidized and form low concentrations of peptide aldehydes, which then act as inhibitors of caspase-3. This type of photoactivation may be utilized in future photopharmacology experiments to form protease inhibitors at a precise time and location.
Keywords: chemical probes; click chemistry; inhibitors; photoactivation; photocrosslinkers; proteases; triazoles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

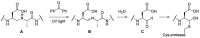
Similar articles
-
Peptide-based covalent inhibitors of MALT1 paracaspase.Bioorg Med Chem Lett. 2019 Jun 1;29(11):1336-1339. doi: 10.1016/j.bmcl.2019.03.046. Epub 2019 Mar 29. Bioorg Med Chem Lett. 2019. PMID: 30954428
-
Controlled Inhibition of Apoptosis by Photoactivatable Caspase Inhibitors.Cell Chem Biol. 2020 Nov 19;27(11):1434-1440.e10. doi: 10.1016/j.chembiol.2020.08.001. Epub 2020 Aug 18. Cell Chem Biol. 2020. PMID: 32814013
-
Ac-tLeu-Asp-H is the minimal and highly effective human caspase-3 inhibitor: biological and in silico studies.Amino Acids. 2015 Jan;47(1):153-62. doi: 10.1007/s00726-014-1855-3. Epub 2014 Oct 21. Amino Acids. 2015. PMID: 25331424
-
1,2,3-Triazoles as Biomimetics in Peptide Science.Molecules. 2021 May 14;26(10):2937. doi: 10.3390/molecules26102937. Molecules. 2021. PMID: 34069302 Free PMC article. Review.
-
Synthesis and enzyme inhibitory activities of novel peptide isosteres.Curr Med Chem. 2002 Dec;9(24):2243-70. doi: 10.2174/0929867023368692. Curr Med Chem. 2002. PMID: 12470245 Review.
Cited by
-
1,4-Disubstituted 1,2,3-Triazoles as Amide Bond Surrogates for the Stabilisation of Linear Peptides with Biological Activity.Molecules. 2020 Aug 6;25(16):3576. doi: 10.3390/molecules25163576. Molecules. 2020. PMID: 32781656 Free PMC article. Review.
-
Efficient Strategy to Design Protease Inhibitors: Application to Enterovirus 71 2A Protease.ACS Bio Med Chem Au. 2022 Apr 19;2(4):437-449. doi: 10.1021/acsbiomedchemau.2c00001. eCollection 2022 Aug 17. ACS Bio Med Chem Au. 2022. PMID: 37102167 Free PMC article.
References
-
- Goncalves P., Verhelst S.H.L. Chemical Probes Targeting Proteases for Imaging and Diagnostics in Cancer. 1st ed. John Wiley & Sons; Hoboken, NJ, USA: 2018. pp. 351–375.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

