Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties
- PMID: 30626077
- PMCID: PMC6337346
- DOI: 10.3390/ijms20010213
Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties
Abstract
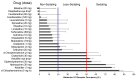
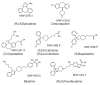
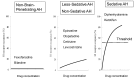
Antihistamines targeting the histamine H₁ receptor play an important role in improving and maintaining the quality of life of patients with allergic rhinitis. For more effective and safer use of second-generation drugs, which are recommended by various guidelines, a classification based on their detailed characteristics is necessary. Antihistamines for first-line therapy should not have central depressant/sedative activities. Sedative properties (drowsiness and impaired performance) are associated with the inhibition of central histamine neurons. Brain H₁ receptor occupancy (H₁RO) is a useful index shown to be correlated with indices based on clinical findings. Antihistamines are classified into non-sedating (<20%), less-sedating (20⁻50%), and sedating (≥50%) groups based on H₁RO. Among the non-sedating group, fexofenadine and bilastine are classified into "non-brain-penetrating antihistamines" based on the H₁RO. These two drugs have many common chemical properties. However, bilastine has more potent binding affinity to the H₁ receptor, and its action tends to last longer. In well-controlled studies using objective indices, bilastine does not affect psychomotor or driving performance even at twice the usual dose (20 mg). Upon selecting antihistamines for allergic rhinitis, various situations should be taken into our consideration. This review summarizes that the non-brain-penetrating antihistamines should be chosen for the first-line therapy of mild allergic rhinitis.
Keywords: H1 receptor occupancy; allergic rhinitis; antihistamine; bilastine; fexofenadine; non-brain-penetrating.
Conflict of interest statement
The corresponding author (K.Y.) received research grants from Meiji Seika Pharma, Glaxo Smith Kline, Taiho Pharma, Sanofi, Kyorin Pharmaceutical Company. In the last three years, K.Y. had received honoraria from manufactures of 2nd generation antihistamines, including Sanofi, GlaxoSmithKline, Kyowa-Kirin, Taiho Pharma, Meiji Seika Pharma, and Mitsubishi Tanabe Pharma. Other authors have similar conflicts of interest. The contents regarding all aspects of the review were made by all academic authors without consulting with the respective pharmaceutical companies. The role of author from the pharmaceutical industry (K.I.) is the confirmation of description from the points of compilation for approved drug package information.
Figures


Similar articles
-
Bilastine safety in drivers who need antihistamines: new evidence from high-speed simulator driving test on allergic patients.Eur Rev Med Pharmacol Sci. 2018 Feb;22(3):820-828. doi: 10.26355/eurrev_201802_14318. Eur Rev Med Pharmacol Sci. 2018. PMID: 29461615 Clinical Trial.
-
The Impact of Bilastine on Symptoms of Allergic Rhinitis and Chronic Urticaria: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Am J Rhinol Allergy. 2022 Sep;36(5):684-694. doi: 10.1177/19458924221097449. Epub 2022 May 20. Am J Rhinol Allergy. 2022. PMID: 35593100
-
How bilastine is used to treat allergic rhinitis and urticaria in children.Immunotherapy. 2022 Jan;14(1):77-89. doi: 10.2217/imt-2021-0251. Epub 2021 Dec 1. Immunotherapy. 2022. PMID: 34850647 Clinical Trial.
-
The concept of receptor occupancy to predict clinical efficacy: a comparison of second generation H1 antihistamines.Allergy Asthma Proc. 2009 Jul-Aug;30(4):366-76. doi: 10.2500/aap.2009.30.3226. Epub 2009 Mar 30. Allergy Asthma Proc. 2009. PMID: 19335943 Review.
-
[Oral second generation antihistamines in allergic rhinitis].Laryngorhinootologie. 2005 Jan;84(1):30-41. doi: 10.1055/s-2004-826000. Laryngorhinootologie. 2005. PMID: 15647976 Review. German.
Cited by
-
Efficacy and safety of bilastine vs. levocetirizine for the treatment of chronic idiopathic urticaria: A multicenter, double-blind, double-dummy, phase III, non-inferiority, randomized clinical trial.Chin Med J (Engl). 2024 Jun 20;137(12):1480-1482. doi: 10.1097/CM9.0000000000003071. Epub 2024 Apr 1. Chin Med J (Engl). 2024. PMID: 38557589 Free PMC article. Clinical Trial. No abstract available.
-
The safety and tolerability profile of bilastine for chronic urticaria in children.Clin Transl Allergy. 2019 Oct 23;9:55. doi: 10.1186/s13601-019-0294-3. eCollection 2019. Clin Transl Allergy. 2019. PMID: 31660121 Free PMC article. Review.
-
Pathogenesis of allergic diseases and implications for therapeutic interventions.Signal Transduct Target Ther. 2023 Mar 24;8(1):138. doi: 10.1038/s41392-023-01344-4. Signal Transduct Target Ther. 2023. PMID: 36964157 Free PMC article. Review.
-
[Clinical observation of nasal spray allergen blocker combined with antihistamines in treatment of allergic rhinitis children].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020 Jan;34(1):60-63. doi: 10.13201/j.issn.1001-1781.2020.01.015. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020. PMID: 32086901 Free PMC article. Clinical Trial. Chinese.
-
Inhibitory effect of rosmarinic acid on IgE-trigged mast cell degranulation in vitro and in vivo.Mol Biol Rep. 2024 Jan 25;51(1):194. doi: 10.1007/s11033-023-09164-z. Mol Biol Rep. 2024. PMID: 38270683
References
-
- Bousquet J., Khaltaev N., Cruz A.A., Denburg J., Fokkens W.J., Togias A., Zuberbier T., Baena-Cagnani C.E., Canonica G.W., van Weel C., et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(Suppl. 86):8–160. - PubMed
-
- Wang D., Clement P., Smitz J., De Waele M., Derde M.P. Correlations between complaints, inflammatory cells and mediator concentrations in nasalsecretions after nasal allergen challenge and during natural allergen exposure. Int. Arch. Allergy Immunol. 1995;106:278–285. doi: 10.1159/000236855. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

