Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties
- PMID: 30626077
- PMCID: PMC6337346
- DOI: 10.3390/ijms20010213
Antihistamines for Allergic Rhinitis Treatment from the Viewpoint of Nonsedative Properties
Abstract
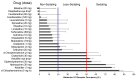
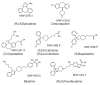
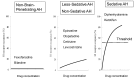
Antihistamines targeting the histamine H₁ receptor play an important role in improving and maintaining the quality of life of patients with allergic rhinitis. For more effective and safer use of second-generation drugs, which are recommended by various guidelines, a classification based on their detailed characteristics is necessary. Antihistamines for first-line therapy should not have central depressant/sedative activities. Sedative properties (drowsiness and impaired performance) are associated with the inhibition of central histamine neurons. Brain H₁ receptor occupancy (H₁RO) is a useful index shown to be correlated with indices based on clinical findings. Antihistamines are classified into non-sedating (<20%), less-sedating (20⁻50%), and sedating (≥50%) groups based on H₁RO. Among the non-sedating group, fexofenadine and bilastine are classified into "non-brain-penetrating antihistamines" based on the H₁RO. These two drugs have many common chemical properties. However, bilastine has more potent binding affinity to the H₁ receptor, and its action tends to last longer. In well-controlled studies using objective indices, bilastine does not affect psychomotor or driving performance even at twice the usual dose (20 mg). Upon selecting antihistamines for allergic rhinitis, various situations should be taken into our consideration. This review summarizes that the non-brain-penetrating antihistamines should be chosen for the first-line therapy of mild allergic rhinitis.
Keywords: H1 receptor occupancy; allergic rhinitis; antihistamine; bilastine; fexofenadine; non-brain-penetrating.
Conflict of interest statement
The corresponding author (K.Y.) received research grants from Meiji Seika Pharma, Glaxo Smith Kline, Taiho Pharma, Sanofi, Kyorin Pharmaceutical Company. In the last three years, K.Y. had received honoraria from manufactures of 2nd generation antihistamines, including Sanofi, GlaxoSmithKline, Kyowa-Kirin, Taiho Pharma, Meiji Seika Pharma, and Mitsubishi Tanabe Pharma. Other authors have similar conflicts of interest. The contents regarding all aspects of the review were made by all academic authors without consulting with the respective pharmaceutical companies. The role of author from the pharmaceutical industry (K.I.) is the confirmation of description from the points of compilation for approved drug package information.
Figures


References
-
- Bousquet J., Khaltaev N., Cruz A.A., Denburg J., Fokkens W.J., Togias A., Zuberbier T., Baena-Cagnani C.E., Canonica G.W., van Weel C., et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(Suppl. 86):8–160. - PubMed
-
- Wang D., Clement P., Smitz J., De Waele M., Derde M.P. Correlations between complaints, inflammatory cells and mediator concentrations in nasalsecretions after nasal allergen challenge and during natural allergen exposure. Int. Arch. Allergy Immunol. 1995;106:278–285. doi: 10.1159/000236855. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

