Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration
- PMID: 30626110
- PMCID: PMC6337628
- DOI: 10.3390/ijms20010210
Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration
Abstract
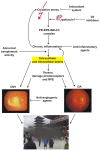
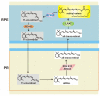
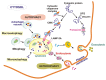
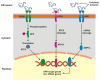
Age-related macular degeneration (AMD) is a complex eye disease with many pathogenesis factors, including defective cellular waste management in retinal pigment epithelium (RPE). Main cellular waste in AMD are: all-trans retinal, drusen and lipofuscin, containing unfolded, damaged and unneeded proteins, which are degraded and recycled in RPE cells by two main machineries-the ubiquitin-proteasome system (UPS) and autophagy. Recent findings show that these systems can act together with a significant role of the EI24 (etoposide-induced protein 2.4 homolog) ubiquitin ligase in their action. On the other hand, E3 ligases are essential in both systems, but E3 is degraded by autophagy. The interplay between UPS and autophagy was targeted in several diseases, including Alzheimer disease. Therefore, cellular waste clearing in AMD should be considered in the context of such interplay rather than either of these systems singly. Aging and oxidative stress, two major AMD risk factors, reduce both UPS and autophagy. In conclusion, molecular mechanisms of UPS and autophagy can be considered as a target in AMD prevention and therapeutic perspective. Further work is needed to identify molecules and effects important for the coordination of action of these two cellular waste management systems.
Keywords: age-related macular degeneration; autophagy; cellular waste elimination; mitophagy; proteostasis; ubiquitin-proteasome system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration.Prog Retin Eye Res. 2016 Mar;51:69-89. doi: 10.1016/j.preteyeres.2015.09.002. Epub 2015 Sep 4. Prog Retin Eye Res. 2016. PMID: 26344735 Free PMC article. Review.
-
Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD.Autophagy. 2014;10(11):1989-2005. doi: 10.4161/auto.36184. Autophagy. 2014. PMID: 25484094 Free PMC article.
-
Crosstalk Between the Autophagy-Lysosome Pathway and the Ubiquitin-Proteasome Pathway in Retinal Pigment Epithelial Cells.Curr Mol Med. 2016;16(5):487-95. doi: 10.2174/1566524016666160429121606. Curr Mol Med. 2016. PMID: 27132793
-
Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration.Prog Retin Eye Res. 2020 Nov;79:100858. doi: 10.1016/j.preteyeres.2020.100858. Epub 2020 Apr 13. Prog Retin Eye Res. 2020. PMID: 32298788 Free PMC article. Review.
-
Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics).Aging Cell. 2012 Feb;11(1):1-13. doi: 10.1111/j.1474-9726.2011.00752.x. Epub 2011 Nov 15. Aging Cell. 2012. PMID: 21967227 Free PMC article.
Cited by
-
The intertwining of Zn-finger motifs and abiotic stress tolerance in plants: Current status and future prospects.Front Plant Sci. 2023 Jan 4;13:1083960. doi: 10.3389/fpls.2022.1083960. eCollection 2022. Front Plant Sci. 2023. PMID: 36684752 Free PMC article. Review.
-
RING Zinc Finger Proteins in Plant Abiotic Stress Tolerance.Front Plant Sci. 2022 Apr 14;13:877011. doi: 10.3389/fpls.2022.877011. eCollection 2022. Front Plant Sci. 2022. PMID: 35498666 Free PMC article. Review.
-
Epoxomicin, a Selective Proteasome Inhibitor, Activates AIM2 Inflammasome in Human Retinal Pigment Epithelium Cells.Antioxidants (Basel). 2022 Jun 28;11(7):1288. doi: 10.3390/antiox11071288. Antioxidants (Basel). 2022. PMID: 35883779 Free PMC article.
-
HIF-1α-dependent upregulation of angiogenic factors by mechanical stimulation in retinal pigment epithelial cells.Dis Model Mech. 2024 Apr 1;17(4):dmm050640. doi: 10.1242/dmm.050640. Epub 2024 May 1. Dis Model Mech. 2024. PMID: 38691000 Free PMC article.
-
Urban Aerosol Particulate Matter Promotes Necrosis and Autophagy via Reactive Oxygen Species-Mediated Cellular Disorders that are Accompanied by Cell Cycle Arrest in Retinal Pigment Epithelial Cells.Antioxidants (Basel). 2021 Jan 20;10(2):149. doi: 10.3390/antiox10020149. Antioxidants (Basel). 2021. PMID: 33498524 Free PMC article.
References
-
- Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

