Failure to eliminate a phosphorylated glucose analog leads to neutropenia in patients with G6PT and G6PC3 deficiency
- PMID: 30626647
- PMCID: PMC6347702
- DOI: 10.1073/pnas.1816143116
Failure to eliminate a phosphorylated glucose analog leads to neutropenia in patients with G6PT and G6PC3 deficiency
Abstract
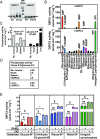
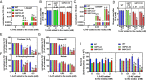
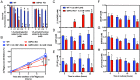
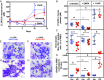
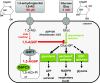
Neutropenia represents an important problem in patients with genetic deficiency in either the glucose-6-phosphate transporter of the endoplasmic reticulum (G6PT/SLC37A4) or G6PC3, an endoplasmic reticulum phosphatase homologous to glucose-6-phosphatase. While affected granulocytes show reduced glucose utilization, the underlying mechanism is unknown and causal therapies are lacking. Using a combination of enzymological, cell-culture, and in vivo approaches, we demonstrate that G6PT and G6PC3 collaborate to destroy 1,5-anhydroglucitol-6-phosphate (1,5AG6P), a close structural analog of glucose-6-phosphate and an inhibitor of low-KM hexokinases, which catalyze the first step in glycolysis in most tissues. We show that 1,5AG6P is made by phosphorylation of 1,5-anhydroglucitol, a compound normally present in human plasma, by side activities of ADP-glucokinase and low-KM hexokinases. Granulocytes from patients deficient in G6PC3 or G6PT accumulate 1,5AG6P to concentrations (∼3 mM) that strongly inhibit hexokinase activity. In a model of G6PC3-deficient mouse neutrophils, physiological concentrations of 1,5-anhydroglucitol caused massive accumulation of 1,5AG6P, a decrease in glucose utilization, and cell death. Treating G6PC3-deficient mice with an inhibitor of the kidney glucose transporter SGLT2 to lower their blood level of 1,5-anhydroglucitol restored a normal neutrophil count, while administration of 1,5-anhydroglucitol had the opposite effect. In conclusion, we show that the neutropenia in patients with G6PC3 or G6PT mutations is a metabolite-repair deficiency, caused by a failure to eliminate the nonclassical metabolite 1,5AG6P.
Keywords: 1,5-anhydroglucitol; SLGT2 inhibitors; glucose-6-phosphatase-β; metabolite repair; neutropenia.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Martin CC, et al. Identification and characterization of a human cDNA and gene encoding a ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein. J Mol Endocrinol. 2002;29:205–222. - PubMed
-
- Boustead JN, et al. Identification and characterization of a cDNA and the gene encoding the mouse ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein. J Mol Endocrinol. 2004;32:33–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

